Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity
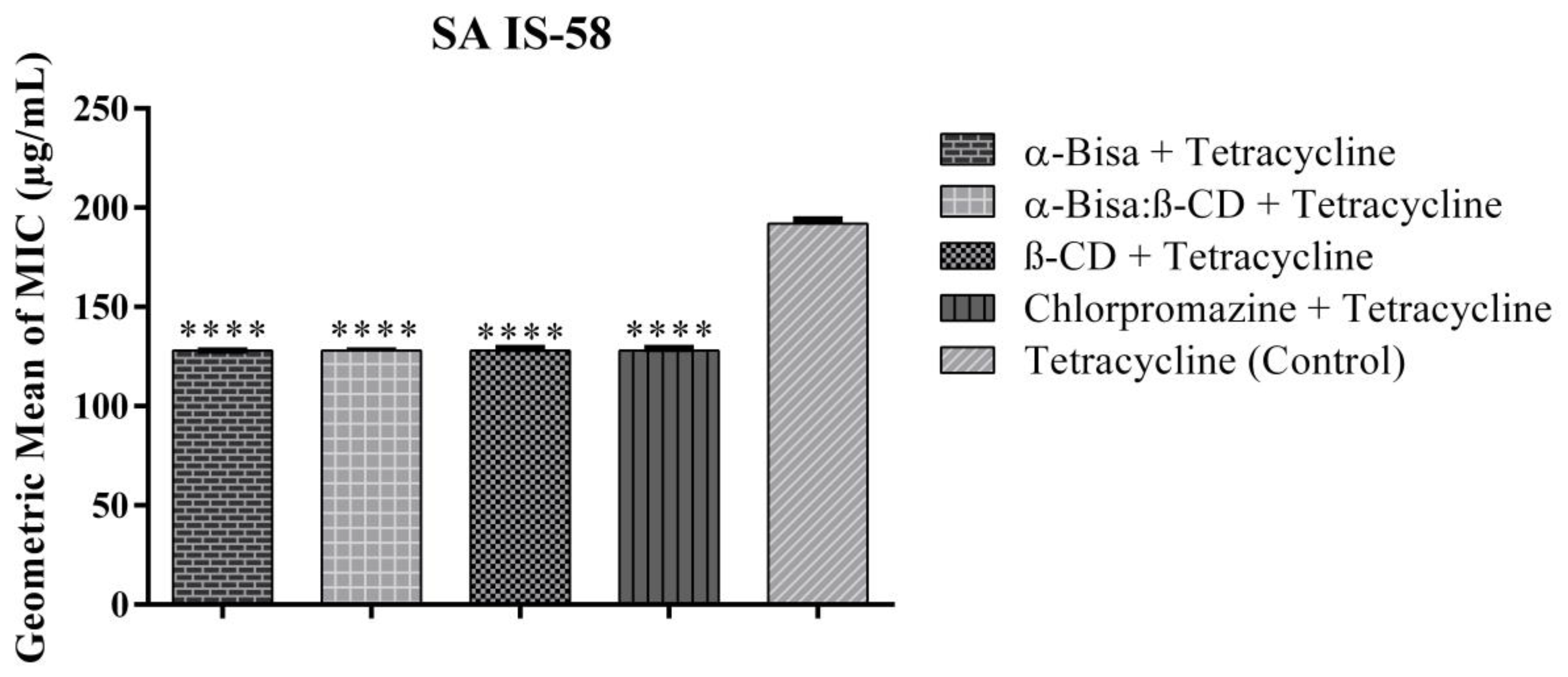
2.2. Inhibitory Effect over Pumps by MIC Reduction
3. Materials and Methods
3.1. Bioactive Compounds and Complexation
3.2. Culture Media
3.3. Bacterial Strains
3.4. Drugs, Pump Inhibitors, and Reagents
3.5. Determination of the Minimum Inhibitory Concentration (MIC)
3.6. Evaluation of Efflux Pump Inhibition by a Modulating Effect
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barbosa, T.M.; Levy, S.B. The impact of antibiotic use on resistance development and persistence. Drug Resist. Updates 2000, 3, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Mukherjee, M.M.; Varela, M.F. Modulation of bacterial multidrug resistance efflux pumps of the major facilitator superfamily. Int. J. Bacteriol. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, N.J.; Piddock, L.J. Antibacterial efflux systems. Microbiologia. (Madr. Spain) 1997, 13, 285–300. [Google Scholar]
- Sun, J.; Deng, Z.; Yan, A. Bacterial multidrug efflux pumps: Mechanisms, physiology and pharmacological exploitations. Biochem. Biophys. Res. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.M.A.; Piddock, L.J.V. How to measure export via bacterial multidrug resistance efflux pumps. Mbio 2016, 7, e00840-16. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, S.; Oluwatuyi, M.; Kaatz, G.W. A novel inhibitor of multidrug efflux pumps in Staphylococcus Aureus. J. Antimicrob. Chemother. 2013, 51, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Piddock, L.J.V. Multidrug-resistance efflux pumps? not just for resistance. Nat. Rev. Microbiol. 2006, 4, 629–636. [Google Scholar] [CrossRef]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Pages, J.M.; Amaral, L. Mechanisms of drug efflux and strategies to combat them: Challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2009, 1794, 826–833. [Google Scholar] [CrossRef]
- Bezerra, J.W.A.; Costa, A.R.; da Silva, M.A.P.; Rocha, M.I.; Boligon, A.A.; da Rocha, J.B.T.; Barros, L.M.; Kamdem, J.P. Chemical composition and toxicological evaluation of Hyptis suaveolens (L.) Poiteau (LAMIACEAE) in Drosophila melanogaster and Artemia salina. S. Afr. J. Bot. 2017, 113, 437–442. [Google Scholar] [CrossRef]
- Filho, C.V.; Yunes, R.A. Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais. Conceitos sobre modificação estrutural para otimização da atividade. Quím. Nova 1998, 21, 99–105. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2006, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegos, G.P.; Mark, H.; Strouse, J.; Mohiuddin, M.T.K.; Bologa, G.C.; Larry, A.S. Microbial efflux pump inhibition: Tactics and strategies. Curr. Pharm. Des. 2011, 17, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Rocha, N.F.M.; Oliveira, G.V.; Araújo, F.Y.R.; Rios, E.R.V.; Carvalho, A.M.R.; Vasconcelos, L.F.; Macêdo, D.S.; Soares, P.M.G.; Sousa, D.P.S.; Sousa, F.C.F. (−)-α-Bisabolol-induced gastroprotection is associated with reduction in lipid peroxidation, superoxide dismutase activity and neutrophil migration. Eur. J. Pharm. Sci. 2011, 44, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.F.G.; Colares, A.V.; Nonato, C.D.F.A.; Galvão-Rodrigues, F.F.; Mota, M.L.; Morais-Braga, M.F.B.; Costa, J.G.M. In vitro antimicrobial activity of the essential oil from Vanillosmopsis arborea Barker (Asteraceae) and its major constituent, α-bisabolol. Microb. Pathog. 2018, 125, 144–149. [Google Scholar] [CrossRef]
- Russell, K.; Jacob, S.E. Bisabolol. Dermat 2010, 21, 57–58. [Google Scholar] [CrossRef]
- Bhatia, S.P.; McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on alpha-bisabolol. Food Chem. Toxicol. 2008, 46, 72–76. [Google Scholar] [CrossRef]
- CAVALIERI, E.; Mariotto, S.; Fabrizi, C.; Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophs. Res. Commun. 2004, 315, 589–594. [Google Scholar] [CrossRef]
- Corpas-López, V.; Merino-Espinosa, G.; Acedo-Sánchez, C.; Díaz-Sáez, V.; Navarro-Moll, M.C.; Morillas-Márquez, F.; Martín-Sánchez, J. Effectiveness of the sesquiterpene (-)-α-bisabolol in dogs with naturally acquired canine leishmaniosis: An exploratory clinical trial. Vet. Res. Commun. 2018, 42, 121–130. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The Biological Activities of 20 Nature Identical Essential Oil Constituents. J. Essent. Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. A review of the application and pharmacological properties of α-bisabolol and α-bisabolol-rich oils. J. Am. Oil Chem. Soc. 2010, 87, 1–7. [Google Scholar] [CrossRef]
- Yatsu, F.K.J.F.; Koester, L.S.; Lula, I.; Passos, J.J.; Sinisterra, R.; Bassani, V.L. Multiple complexation of cyclodextrin with soy isoflavones present in an enriched fraction. Carbohydr. Polym. 2013, 98, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, M.A.F.O.; Júnior, C.S.N.; Santos, H.F. Análise estrutural de ciclodextrinas: Um estudo comparativo entre métodos teóricos clássicos e quânticos. Quím. Nova 2004, 6, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Saltão, R.; Veiga, F. Ciclodextrinas em novos sistemas terapêuticos. Brazil. J. Pharm. Sci. 2001, 37, 2–16. [Google Scholar]
- Oliveira, R.; Santos, D.; Coelho, P. Ciclodextrinas: Formação de complexos e sua aplicação farmacêutica. R. Fac. De Ciênc. Da Saúde 2009, 6, 70–83. [Google Scholar]
- Houghton, P.J.; Howes, M.J.; Lee, C.C.; Steventon, G. Uses and abuses of in vitro tests in ethnopharmacology: Visualizing an elephant. J. Ethnopharmacol. 2007, 110, 391–400. [Google Scholar] [CrossRef]
- Oliveira, F.S.; Freitas, T.S.; Cruz, R.P.; Costa, M.S.; Pereira, R.L.S.; Quintans-Júnior, L.J.; Andrade, T.A.; Menezes, P.P.; Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef]
- Andrade, T.A.; Freitas, T.S.; Araújo, F.O.A.; Menezes, P.P.; Doria, A.A.; Rabelo, A.S.; Quintans-Júnior, L.J.; Santos, M.R.V.; Bezerra, D.P.; Serafini, M.R.; et al. Physico-chemical characterization and antibacterial activity of inclusion complexes of Hyptis martiusii Benth essential oil in β-cyclodextrin. Biomed. Pharmacother. 2017, 89, 201–207. [Google Scholar] [CrossRef]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [Green Version]
- Bailey, A.M.; Paulsen, I.T.; Piddock, L.J.V. RamA confers multidrug resistance in Salmonella enterica via increased expression of acrB, which is inhibited by chlorpromazine. Antimicrob. Agents Chemother. 2008, 52, 3604–3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.; Wagner, D.; Viveiros, M.; Sampaio, D.; Couto, I.; Vavra, M.; Kern, W.V.; Amaral, L. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J. Antimicrob. Chemother. 2008, 61, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie 2005, 87, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Limaverde, P.W.; Campina, F.F.; Cunha, F.A.B.; Crispim, F.D.; Figueredo, F.G.; Lima, L.F.; Oliveira-Tintino, C.D.M.; Matos, Y.M.L.; Morais-Braga, M.F.B.; Menezes, I.R.A.; et al. Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 2017, 109, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, S.; Xu, L.; Fu, W.; Gibbons, S. Sesquiterpenoids with Anti-MDR Staphylococcus aureus Activities from Ferula ferulioides. Chem. Biodivers. 2015, 12, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
- Simões, M.; Silvia, R.; Manuel, C.A.; Vierira, M.J. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008, 4, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, H.F.D. Nanoagregados Baseados Em Ciclodextrinas Em Associação Com a Tetraciclina: Caracterização Fisico-Química E Avaliação Antimicrobiana. Dissertação Mestrado em Odontologia, Universidade Federal de Minas Gerais, Belo horizonte-MG, Brasil, 2007; p. 82. [Google Scholar]
- Waleczek, K.J.; Marques, C.H.M.; Hempel, B.; Schimidt, P.C.S. Phase solubility studies of pure (−)-α-bisabolol and camomile essential oil with β-cyclodextrin. Eur. J. Pharm. Biopharm. 2003, 55, 247–251. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 107–3113. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; CLSI Document M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Viveiros, M.; Martins, M.; Couto, I.; Rodrigues, L.; Spengler, G.; Martins, A.; Kristiansen, J.E.; Molnar, J.; Amaral, L. New methods for the identification of efflux mediated MDR bacteria, genetic assessment of regulators and efflux pump constituents, characterization of efflux systems and screening for inhibitors of efflux pumps. Curr. Drug Targets 2008, 9, 760–778. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef] [PubMed]

| Strain | SA IS-58 | SA 1199B |
|---|---|---|
| α-Bisabolol | ≥1024 | ≥1024 |
| α-Bisabolol/β-CD | ≥1024 | ≥1024 |
| β-Cyclodextrin | ≥1024 | ≥1024 |
| Chlorpromazine | 128 | 128 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira da Cruz, R.; Sampaio de Freitas, T.; do Socorro Costa, M.; Lucas dos Santos, A.T.; Ferreira Campina, F.; Pereira, R.L.S.; Bezerra, J.W.A.; Quintans-Júnior, L.J.; De Souza Araújo, A.A.; De Siqueira Júnior, J.P.; et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiotics 2020, 9, 28. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010028
Pereira da Cruz R, Sampaio de Freitas T, do Socorro Costa M, Lucas dos Santos AT, Ferreira Campina F, Pereira RLS, Bezerra JWA, Quintans-Júnior LJ, De Souza Araújo AA, De Siqueira Júnior JP, et al. Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains. Antibiotics. 2020; 9(1):28. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010028
Chicago/Turabian StylePereira da Cruz, Rafael, Thiago Sampaio de Freitas, Maria do Socorro Costa, Antonia Thassya Lucas dos Santos, Fábia Ferreira Campina, Raimundo Luiz Silva Pereira, José Weverton Almeida Bezerra, Lucindo José Quintans-Júnior, Adriano Antunes De Souza Araújo, José Pinto De Siqueira Júnior, and et al. 2020. "Effect of α-Bisabolol and Its β-Cyclodextrin Complex as TetK and NorA Efflux Pump Inhibitors in Staphylococcus aureus Strains" Antibiotics 9, no. 1: 28. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010028







