Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial
Abstract
:1. Introduction
2. Study Results
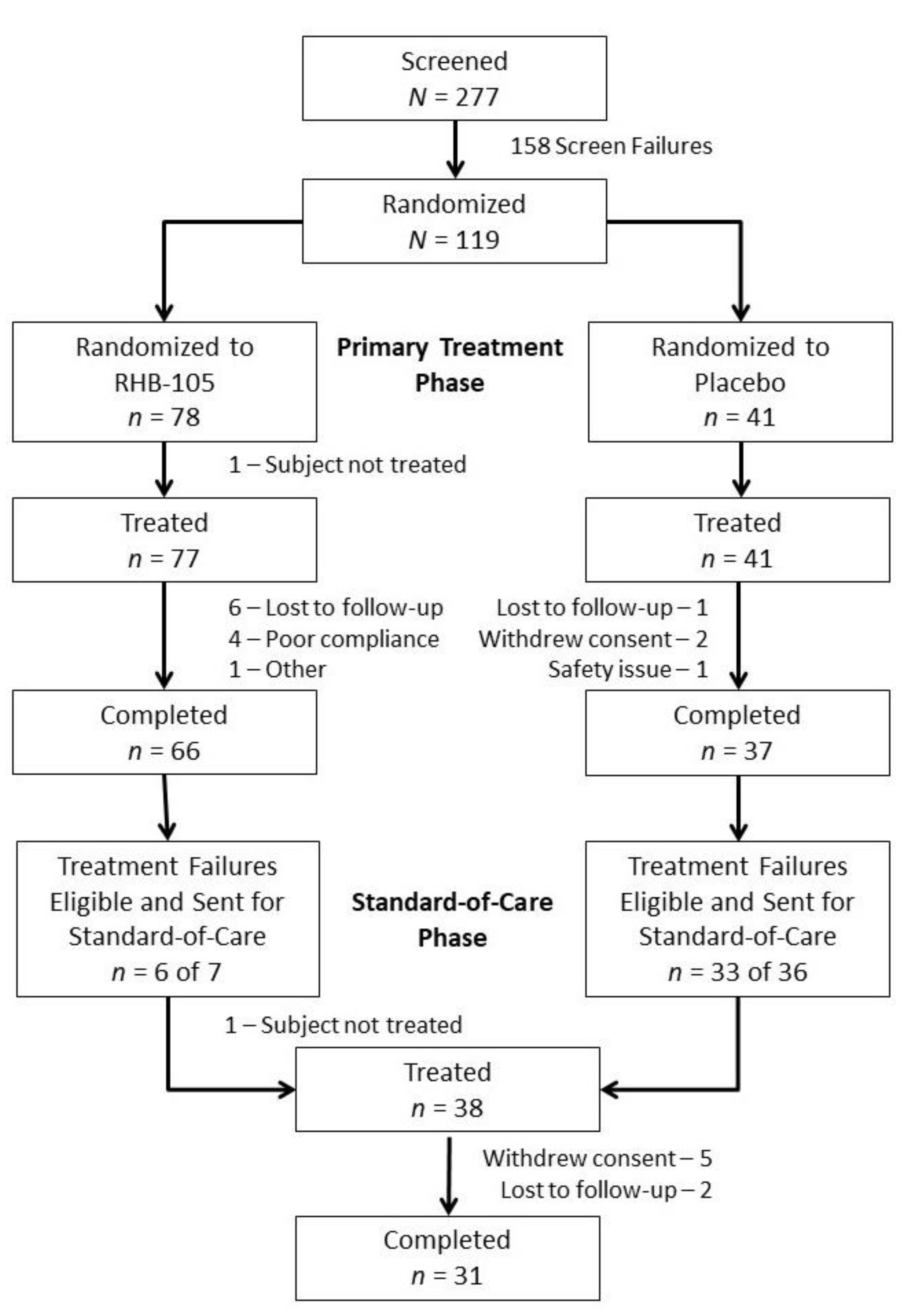
2.1. Participants
2.2. Treatment Efficacy Results
2.3. CYP2C19 Genotyping
2.4. Physician-Selected Standard-of-Care Efficacy Results
2.5. Safety Results
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Participant Eligibility
4.3. Study Design
4.4. Study Drug and Concomitant Medications
4.5. Efficacy and Safety Assessments
4.6. Statistical Methods
4.6.1. Efficacy Analysis
4.6.2. Exploratory Efficacy Analyses
4.7. Data Sharing Statement
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- IARC Working. Group on the Evaluation of Carcinogenic Risks to Humans Schistosomes, liver flukes and Helicobacter pylori. Lyon, 7–14 June 1994. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Kumar, S.; Metz, D.C.; Ellenberg, S.; Kaplan, D.E.; Goldberg, D.S. Risk factors and incidence of gastric cancer after detection of Helicobacter pylori infection: A large cohort study. Gastroenterology 2020, 158, 527–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilay, E.J.; Fagan, R.P. Chapter 3: Infectious Diseases Related to Travel. In 2012 Yellow Book—Travelers’ Health—CDC; Oxford University Press Inc.: New York, NY, USA, 2011. [Google Scholar]
- Cardenas, V.M.; Mulla, Z.D.; Ortiz, M.; Graham, D.Y. Iron deficiency and Helicobacter pylori infection in the United States. Am. J. Epidemiol. 2006, 163, 127–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, D.Y.; Adam, E.; Reddy, G.T.; Agarwal, J.P.; Agarwal, R.; Evans, D.J.; Malaty, H.M.; Evans, D.G. Seroepidemiology of Helicobacter pylori infection in India. Comparison of developing and developed countries. Dig. Dis Sci. 1991, 36, 1084–1088. [Google Scholar] [CrossRef]
- Wannmacher, L. Review of the evidence for H. pylori treatment regimens: Section 17.1 (Review)—Adults and Children. 18th Expert Committee on the Selection and Use of Essential Medicines; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Everhart, J.E.; Kruszon-Moran, D.; Perez-Perez, G.I.; Tralka, T.S.; McQuillan, G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J. Infect. Dis. 2000, 181, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Dang, B.N.; Graham, D.Y. Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 383–384. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef] [Green Version]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration, Health and Human Services. Establishing a list of qualifying pathogens under the Food and Drug Administration Safety and Innovation Act. Federal Register, 5 June 2014. Available online: https://www.federalregister.gov/documents/2014/06/05/2014-13023/establishing-a-list-of-qualifying-pathogens-under-the-food-and-drug-administration-safety-and-innocation-act (accessed on 5 October 2019).
- Piccolomini, R.; Di Bonaventura, G.; Picciani, C.; Laterza, F.; Vecchiet, J.; Neri, M. In vitro activity of clarithromycin against intracellular Helicobacter pylori. Antimicrob. Agents Chemother. 2001, 45, 1568–1571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, C.; Burucoa, C.; Lehours, P.; Tran, C.T.; Leleu, A.; Raymond, J. Molecular characterization of Helicobacter pylori resistance to rifamycins. Helicobacter 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Heep, M.; Beck, D.; Bayerdorffer, E.; Lehn, N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori. Antimicrob. Agents Chemother. 1999, 43, 1497–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perri, F.; Festa, V.; Andriulli, A. Treatment of antibiotic-resistant Helicobacter pylori [letter]. N. Engl. J. Med. 1998, 339, 53. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Di Campli, E.; Di Bartolomeo, S.; Cataldi, V.; Marzio, L.; Grossi, L.; Ciccaglione, A.F.; Nostro, A.; Cellini, L. In vitro antimicrobial susceptibility of Helicobacter pylori to nine antibiotics currently used in central Italy. Scand. J. Gastroenterol. 2016, 51, 263–269. [Google Scholar] [CrossRef]
- Biernat, M.M.; Poniewierka, E.; Błaszczuk, J.; Czapla, L.; Kempiński, R.; Ksiądzyna, D.; Grabińska, J.; Bińkowska, A.; Megraud, F.; Gościniak, G. Antimicrobial susceptibility of Helicobacter pylori isolates from lower Silesia, Poland. Arch. Med. Sci. 2014, 10, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Kunin, C.M. Antimicrobial activity of rifabutin. Clin. Infect. Dis. 1996, 22, S3–S13. [Google Scholar] [CrossRef]
- Koudriakova, T.; Iatsimirskaia, E.; Tulebaev, S.; Spetie, D.; Utkin, I.; Mullet, D.; Thompson, T.; Vouros, P.; Gerber, N. In vivo disposition and metabolism by liver and enterocyte microsomes of the antitubercular drug rifabutin in rats. J. Pharmacol. Exp. Ther. 1996, 279, 1300–1309. [Google Scholar]
- Gisbert, J.P.; Calvert, X. Review article: Rifabutin in the treatment of refractory Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2012, 35, 209–221. [Google Scholar] [CrossRef]
- Alba, C.; Blanco, A.; Alarcón, T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017, 30, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y. Antibiotic resistance in Helicobacter pylori: Implications for therapy. Gastroenterology 1998, 115, 1272–1277. [Google Scholar] [CrossRef]
- Borody, T.J.; Pang, G.; Wettstein, A.R.; Clancy, R.; Herdman, K.; Surace, R.; Llorente, R.; Ng, C. Efficacy and safety of rifabutin-containing ‘rescue therapy’ for resistant Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2006, 23, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Atherton, J.; Axon, A.T.; Bazzoli, F.; Gensini, G.F.; Gisbert, J.P.; Graham, D.Y.; Rokkas, T.; et al. Management of Helicobacter pylori infection—The Maastricht IV/Florence Consensus Report. Gut 2012, 61, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.; Gisbert, J.P.; O’Morain, C.O.; Ladas, S. Treatment of Helicobacter pylori infection 2015. Helicobacter 2015, 20, 54–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venerito, M.; Krieger, T.; Ecker, T.; Leandro, G.; Malfertheiner, P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 2013, 88, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Canaan, Y.; Maher, J.; Wiener, G.; Hulten, K.G.; Kalfus, I.N. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: A double-blind, randomized, controlled trial. Ann. Intern. Med. 2020, 172, 795–802. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | RHB-105 n = 77 | Placebo n = 41 | Total N = 118 |
|---|---|---|---|
| Age, y, mean ± SD | 46.2 ± 10.56 | 45.8 ± 9.52 | 46.0 ± 10.18 |
| Sex, n (%) | |||
| Female | 49 (63.6%) | 25 (61.0%) | 74 (62.7%) |
| Male | 28 (36.4%) | 16 (39.0%) | 44 (37.3%) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 60 (77.9%) | 35 (85.4%) | 95 (80.5%) |
| Not Hispanic or Latino | 17 (22.1%) | 6 (14.6%) | 23 (19.5%) |
| Race, n (%) | |||
| White | 71 (92.2%) | 38 (92.7%) | 109 (92.4%) |
| Black African heritage or African American | 6 (7.8%) | 3 (7.3%) | 9 (7.6%) |
| ITT a | mITT | PP | |
|---|---|---|---|
| RHB-105 | 76.6% (59/77) | 89.4% (59/66) | 88.9% (56/63) |
| 95% CI | 67.2–86.1% | 82.0–96.8% | 81.1–96.7% |
| Literature-derived Historical Control b | 70% | 70% | 70% |
| P-value, RHB-105 vs. Literature-derived Historical Control | 0.085 | <0.001 | <0.001 |
| Physician-selected Standard-of-Care (Placebo Subjects) | 51.5% (17/33) | 63.0% (17/27) | NA |
| 95% CI | 34.5–68.6% | 44.8–81.1% | |
| P-value, RHB-105 vs. Physician-selected Standard-of-Care | 0.009 | 0.006 | NA |
| Placebo | RHB-105 Treatment Failures | Total | |
|---|---|---|---|
| All Subjects | 17/27 (62.9%) | 2/4 (50.0%) | 19/31 (61.3%) |
| Clarithromycin triple therapy a | 14/23 (60.9%) | 2/4 (50.0%) | 16/27 (59.3%) |
| Metronidazole triple therapy b | 1/2 (50.0%) | 0 | 1/2 (50.0%) |
| Bismuth quadruple therapy c | 2/2 (100.0%) | 0 | 2/2 (100.0%) |
| Adverse Event | RHB-105 n = 77 | Placebo n = 41 | Total N = 118 |
|---|---|---|---|
| All subjects with an adverse event | 39 (50.6%) | 19 (46.3%) | 58 (49.2%) |
| Headache a | 12 (15.6%) | 4 (9.8%) | 16 (13.6%) |
| Diarrhea | 11 (14.3%) | 4 (9.8%) | 15 (12.7%) |
| Chromaturia | 10 (13.0%) | 1 (2.4%) | 11 (9.3%) |
| Rash | 4 (5.2%) | 0 | 4 (5.2%) |
| Nausea | 3 (3.9%) | 1 (2.4%) | 4 (3.4%) |
| Oropharyngeal pain | 3 (3.9%) | 0 | 3 (2.5%) |
| Blood creatine phosphokinase increase | 2 (2.6%) | 1 (2.4%) | 3 (2.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalfus, I.N.; Graham, D.Y.; Riff, D.S.; Panas, R.M. Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial. Antibiotics 2020, 9, 685. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9100685
Kalfus IN, Graham DY, Riff DS, Panas RM. Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial. Antibiotics. 2020; 9(10):685. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9100685
Chicago/Turabian StyleKalfus, Ira N., David Y. Graham, Dennis S. Riff, and Raymond M. Panas. 2020. "Rifabutin-Containing Triple Therapy (RHB-105) for Eradication of Helicobacter pylori: Randomized ERADICATE Hp Trial" Antibiotics 9, no. 10: 685. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9100685




