Effective Photodynamic Inactivation of 26 Escherichia coli Strains with Different Antibiotic Susceptibility Profiles: A Planktonic and Biofilm Study
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Susceptibility Profiles
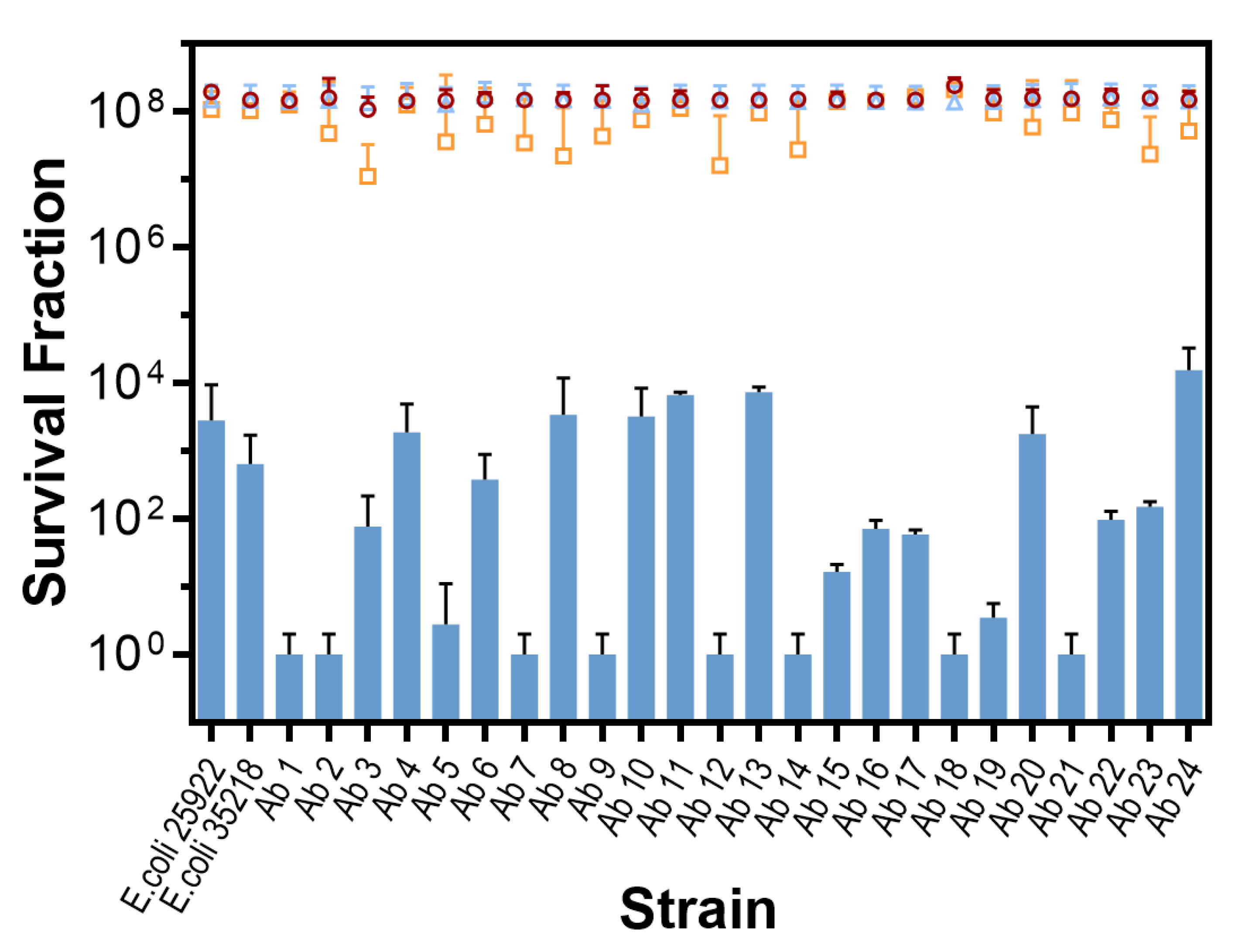
2.2. In Vitro Photodynamic Inactivation of E. coli Growing in Planktonic State
In vitro Photodynamic Inactivation of E. coli Growing in Biofilm
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antibiotic Susceptibility Testing
4.3. Photosensitizer and Light Source
4.4. Photodynamic Inactivation of E. coli Growing in Planktonic State:
4.5. Photodynamic Inactivation of E. coli Growing in Biofilm
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buchholz, K.; Collins, J. The roots—A short history of industrial microbiology and biotechnology. Appl. Microbiol. Biotechnol. 2013, 97, 3747–3762. [Google Scholar] [CrossRef]
- O’Neill. Wellcome Trust. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf/ (accessed on 20 January 2020).
- Cassini, A.; Högberg, D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to conventional antibiotics in the era of antimicrobial resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Maisch, T.; Spannberger, F.; Regensburger, J.; Felgenträger, A.; Bäumler, W. Fast and effective: Intense pulse light photodynamic inactivation of bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 1013–1021. [Google Scholar] [CrossRef]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tomé, J.P.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 571–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanova, N.A.; Brovko, L.Y.; Moore, L.; Pometun, E.; Savitsky, A.P.; Ugarova, N.N.; Griffiths, M.W. Assessment of photodynamic destruction of Escherichia coli O157:H7 and Listeria monocytogenes by using ATP bioluminescence. Appl. Environ. Microbiol. 2003, 69, 6393–6398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, Z.; Faraggi, A.; Savion, N. Ultrastructural damage in photosensitized endothelial cells: Dependence on hematoporphyrin delivery pathways. J. Photochem. Photobiol. B 1992, 14, 359–368. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeter. Biodeg. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Giuliani, F.; Martinelli, M.; Cocchi, A.; Arbia, D.; Fantetti, L.; Roncucci, G. In vitro resistance selection studies of RLP068/Cl, a new Zn(II) phthalocyanine suitable for antimicrobial photodynamic therapy. Antimicrob. Agents Chemother. 2010, 54, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, P.K.; Grinholc, M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sublethal treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. Combination of photodynamic therapy and antimicrobial compounds to treat skin and mucosal infections: A systematic review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; O’Donnell, D.A.; Murthy, N.; Rajagopalan, K.; Michaud, N.; Sherwood, M.E. Polycationic photosensitizer conjugates: Effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 2002, 49, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef]
- Floyd, R.A.; Schneider, J.E., Jr.; Dittmer, D.P. Methylene blue photoinactivation of RNA viruses. Antiviral Res. 2004, 61, 141–151. [Google Scholar] [CrossRef]
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. [Google Scholar] [CrossRef] [Green Version]
- Grinholc, M.; Rapacka-Zdonczyk, A.; Rybak, B.; Szabados, F.; Bielawski, K.P. Multiresistant strains are as susceptible to photodynamic inactivation as their naïve counterparts: Protoporphyrin IX-mediated photoinactivation reveals differences between methicillin-resistant and methicillin-sensitive Staphylococcus aureus strains. Photomed. Laser Surg. 2014, 32, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Caires, C.S.A.; Leal, C.R.B.; Rodrigues, A.C.S.; Lima, A.R.; Silva, C.M.; Ramos, C.A.N.; Chang, M.R.; Arruda, E.J.; Oliveira, S.L.; Nascimento, V.A.; et al. Photoinactivation of mcr-1 positive Escherichia coli. Laser Phys. Lett. 2018, 15. [Google Scholar] [CrossRef]
- Wilson, M.; Yianni, C. Killing of methicillin-resistant Staphylococcus aureus by low-power laser light. J. Med. Microbiol. 1995, 42, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Soncin, M.; Fabris, C.; Busetti, A.; Dei, D.; Nistri, D.; Roncucci, G.; Jori, G. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem. Photobiol. 2002, 10, 815–819. [Google Scholar] [CrossRef]
- Tang, H.M.; Hamblin, M.R.; Yow, C.M. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J. Infect. Chemother. 2007, 13, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisch, T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev. Med. Chem. 2009, 9, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Grinholc, M.; Szramka, B.; Kurlenda, J.; Graczyk, A.; Bielawski, K.P. Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J. Photochem. Photobiol. B 2008, 90, 57–63. [Google Scholar] [CrossRef]
- Parente, T.M.A.L.; Rebouças, E.L.; dos Santos, V.C.V.; Barbosa, F.C.B.; Zaninc, I.C.J. Serratia marcescens resistance profile and its susceptibility to photodynamic antimicrobial chemotherapy. Photodiagnosis Photodyn. Ther. 2016, 14, 185–190. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.M.; Pittet, D. Guideline for hand hygiene in health-care settings: Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Infect. Control Hosp. Epidemiol. 2002, 23, S3–S40. [Google Scholar] [CrossRef]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, photophysical characterization, and photoinduced antibacterial activity of Methylene Blue-loaded amino- and mannose-targeted mesoporous silica nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [Green Version]
- Kashef, N.; Ravaei, S.A.G.; Djavid, G.E. Phototoxicity of phenothiazinium dyes against methicillin-resistant Staphylococcus aureus and multi-drug resistant Escherichia coli. Photodiagnosis Photodyn. Ther. 2012, 9, 11–15. [Google Scholar] [CrossRef]
- Garcez, A.S.; Núñez, S.C.; Baptista, M.S.; Daghastanli, N.A.; Itri, R.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide. Photochem. Photobiol. Sci. 2011, 10, 483–490. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem. Photobiol. Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegos, G.P.; Hamblin, M.R. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob. Agents Chemother. 2006, 50, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rineh, A.; Dolla, N.K.; Ball, A.R.; Magana, M.; Bremner, J.B.; Hamblin, M.R.; Tegos, G.P.; Kelso, M.J. Attaching the NorA efflux pump inhibitor INF55 to Methylene Blue enhances antimicrobial photodynamic inactivation of methicillin-resistant Staphylococcus aureus in vitro and in vivo. ACS Infect Dis. 2017, 3, 756–766. [Google Scholar] [CrossRef] [PubMed]
- McDermott, P.F.; Walker, R.D.; White, D.G. Antimicrobials: Modes of action and mechanisms of resistance. Int. J. Toxicol. 2003, 22, 135–143. [Google Scholar] [CrossRef]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, H.; Lee, S.W.; Carnicelli, J.; Jiang, Z.; Ren, D. Antibiotic susceptibility of Escherichia coli cells during early-stage biofilm formation. J. Bacteriol. 2019, 201, e00034-19. [Google Scholar] [CrossRef] [Green Version]
- Ronqui, M.R.; de Aguiar Coletti, T.M.; de Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B 2016, 158, 122–129. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.S.; Prates, R.A.; de Santi, M.E.; Lopes, R.G.; Bussadori, S.K.; Ferreira, L.R.; Deana, A.M. Photodynamic inactivation of Candida albicans biofilm: Influence of the radiant energy and photosensitizer charge. Photodiagnosis Photodyn. Ther. 2016, 14, 111–114. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard—12th ed.; CLSI document M02-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Naves, P.; del Prado, G.; Huelves, L.; Gracia, M.; Ruiz, V.; Blanco, J.; Dahbi, G.; Blanco, M.; del Carmen Pontea, M.; Soriano, F. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 2008, 45, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Crémet, L.; Corvec, S.; Batard, E.; Auger, M.; Lopez, I.; Pagniez, F.; Dauvergne, S.; Caroff, N. Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diagn. Microbiol. Infect. Dis. 2013, 75, 252–255. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Category | Antibiotic | Bacterial Target | Identification Number of the Resistant Strains | % of Resistant Strains |
|---|---|---|---|---|
| Penicillins | Ampicillin | Cell wall synthesis | 1–7, 9–14, 17, 19, and 20 | 67 |
| Penicillins + β-lactamase inhibitors | Amoxicillin + Clavulanic acid | 21 and 23 | 8 | |
| First-generation Cephalosporins | Cephalotin | 1, 2, 4, 6–10, 14–24 | 79 | |
| Second-generation Cephalosporins | Cefuroxime | 1, 6–8, 14, 17, 19, and 20 | 33 | |
| Third-generation Cephalosporins | Cefotaxime | None of them | 0 | |
| Cephamycins | Aztreonam | 1, 4–9, 12, 14–16, 18, 22, and 24 | 58 | |
| Cefoxitin | None of them | 0 | ||
| Quinolones | Ciprofloxacin | DNA gyrase | None of them | 0 |
| Nalidixic acid | 3, 4–9, 11, 13, 15–20, 22, and 24. | 71 | ||
| Norfloxacin | 4 | 4 | ||
| Macrolides | Azithromycin | 50S subunit of the ribosome | 4, 9, 17, 19, and 20 | 21 |
| Tetracyclines | Tetracycline | 30S subunit of the ribosome | 1–15, 18, 20, 21, 23, and 24. | 83 |
| Aminoglycosides | Gentamycin | 1–4, 6, 7, 9, 10, 13, 14, 17, and 19 | 50 | |
| Phosphoenolpyruvates | Fosfomycin | UDP-N-acetylglucosamine enolpyruvyl transferase | 1, 2, 4, 5, 8–12, 14, 15, 17–20, and 24 | 67 |
| Furantoins | Nitrofurantoin | Various bacterial enzymes and DNA | 2–13, 15–19, 21–24 | 88 |
| Diaminopyrimidine + Sulfamide | Trimethoprim + Sulfamethoxazole | Synthesis of folic acid | 1–5, 8, 11–15, 18, 20, 21, 23, and 24. | 67 |
| Antibiotic | Strains | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| Ampicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||
| Amoxicillin + Clavulanic acid | R | R | ||||||||||||||||||||||
| Cephalotin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | |||||
| Cefuroxime | R | R | R | R | R | R | R | R | ||||||||||||||||
| Cefotaxime | ||||||||||||||||||||||||
| Aztreonam | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||||
| Cefoxitin | ||||||||||||||||||||||||
| Ciprofloxacin | ||||||||||||||||||||||||
| Nalidixic acid | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | |||||||
| Norfloxacin | R | |||||||||||||||||||||||
| Azithromycin | R | R | R | R | R | |||||||||||||||||||
| Tetracycline | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||
| Gentamycin | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||||||
| Fosfomycin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||
| Nitrofurantoin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | |||
| Trimethoprim + Sulfamethoxazole | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | ||||||||
| Number of resistances | 8 | 7 | 6 | 11 | 7 | 8 | 8 | 8 | 9 | 6 | 6 | 6 | 6 | 8 | 7 | 4 | 8 | 7 | 8 | 8 | 5 | 4 | 5 | 7 |
| Multidrug resistant? | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y |
| Antibiotic | Dose/µg | Reference of the Rosco Neo-Sensitabs™ tablets |
|---|---|---|
| Ampicillin | 10 | 567NR 60212 |
| Amoxicillin + Clavulanic acid | 20 + 10 | 567NR 60112 |
| Cephalothin | 30 | 567NR 60612 |
| Cefuroxime | 30 | 567NR 60512 |
| Cefotaxime | 30 | 567NR 63912 |
| Aztreonam | 30 | 567NR 63612 |
| Cefoxitin | 10 | 567NR 62912 |
| Ciprofloxacin | 5 | 567NR 60812 |
| Nalidixic acid | 30 | 567NR 61412 |
| Norfloxacin | 10 | 567NR 76212N |
| Azithromycin | 15 | 567NR 60312 |
| Tetracycline | 30 | 567NR 62012 |
| Gentamycin | 10 | 567NR 61112 |
| Fosfomycin | 200 | 567NR 62312 |
| Nitrofurantoin | 300 | 567NR 62612 |
| Trimethoprim + Sulfamethoxazole | 1.25 + 23.7 | 567NR 62212 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulías, Ò.; McKenzie, G.; Bayó, M.; Agut, M.; Nonell, S. Effective Photodynamic Inactivation of 26 Escherichia coli Strains with Different Antibiotic Susceptibility Profiles: A Planktonic and Biofilm Study. Antibiotics 2020, 9, 98. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030098
Gulías Ò, McKenzie G, Bayó M, Agut M, Nonell S. Effective Photodynamic Inactivation of 26 Escherichia coli Strains with Different Antibiotic Susceptibility Profiles: A Planktonic and Biofilm Study. Antibiotics. 2020; 9(3):98. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030098
Chicago/Turabian StyleGulías, Òscar, Giselle McKenzie, Miquel Bayó, Montserrat Agut, and Santi Nonell. 2020. "Effective Photodynamic Inactivation of 26 Escherichia coli Strains with Different Antibiotic Susceptibility Profiles: A Planktonic and Biofilm Study" Antibiotics 9, no. 3: 98. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9030098






