Tolerance and Persister Formation in Oral Streptococci
Abstract
:1. Introduction
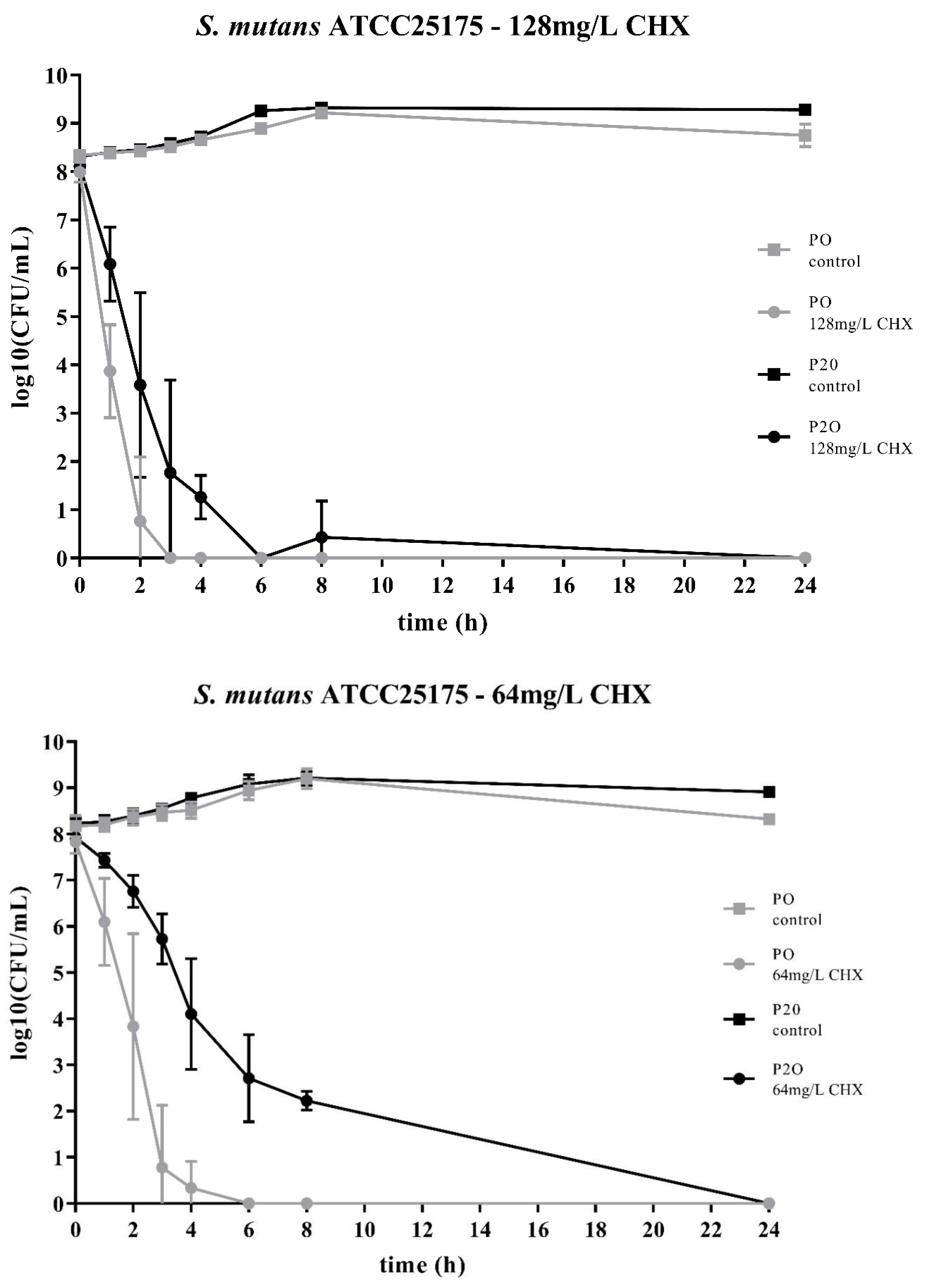
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Test Substance
4.3. Testing of Mutans Streptococci
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Albandar, J.M.; Tinoco, E.M. Global epidemiology of periodontal diseases in children and young persons. Periodontology 2002, 29, 153–176. [Google Scholar] [CrossRef]
- Schurch, E., Jr.; Lang, N.P. Periodontal conditions in Switzerland at the end of the 20th century. Oral Health Prev. Dent. 2004, 2, 359–368. [Google Scholar]
- Offenbacher, S. Periodontal diseases: Pathogenesis. Ann. Periodontol. 1996, 1, 821–878. [Google Scholar] [CrossRef]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Russo, L.L.; Muzio, L.L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rescala, B.; Rosalem, W., Jr.; Teles, R.P.; Fischer, R.G.; Haffajee, A.D.; Socransky, S.S.; Gustafsson, A.; Figueredo, C.M. Immunologic and microbiologic profiles of chronic and aggressive periodontitis subjects. J. Periodontol. 2010, 81, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Indelicato, F.; Ferlito, S. Analysis of Endothelin-1 Concentrations in Individuals with Periodontitis. Sci. Rep. 2020, 10, 1652. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Muraglie, S.; Leonardi, R.; Lo Giudice, A. Assessment of Vitamin C and Antioxidant Profiles in Saliva and Serum in Patients with Periodontitis and Ischemic Heart Disease. Nutrients 2019, 11, 2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paster, B.J.; Olsen, I.; Aas, J.A.; Dewhirst, F.E. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontology 2006, 42, 80–87. [Google Scholar] [CrossRef]
- Zenobia, C.; Hajishengallis, G. Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 2015, 6, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Netuschil, L.; Weiger, R.; Preisler, R.; Brecx, M. Plaque bacteria counts and vitality during chlorhexidine, meridol and listerine mouthrinses. Eur. J. Oral Sci. 1995, 103, 355–361. [Google Scholar] [CrossRef]
- Rohrer, N.; Widmer, A.F.; Waltimo, T.; Kulik, E.M.; Weiger, R.; Filipuzzi-Jenny, E.; Walter, C. Antimicrobial efficacy of 3 oral antiseptics containing octenidine, polyhexamethylene biguanide, or Citroxx: Can chlorhexidine be replaced? Infect. Control Hosp. Epidemiol. 2010, 31, 733–739. [Google Scholar] [CrossRef] [Green Version]
- El Moug, T.; Rogers, D.T.; Furr, J.R.; el-Falaha, B.M.; Russell, A.D. Antiseptic-induced changes in the cell surface of a chlorhexidine-sensitive and a chlorhexidine-resistant strain of Providencia stuartii. J. Antimicrob. Chemother. 1985, 16, 685–689. [Google Scholar] [CrossRef]
- Nakahara, H.; Kozukue, H. Chlorhexidine resistance in Escherichia coli isolated from clinical lesions. Zentralbl Bakteriol Mikrobiol Hyg A 1981, 251, 177–184. [Google Scholar] [CrossRef]
- Nakahara, H.; Kozukue, H. Isolation of chlorhexidine-resistant Pseudomonas aeruginosa from clinical lesions. J. Clin. Microbiol. 1982, 15, 166–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stickler, D.J. Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: A perspective from the study of catheter biofilms. J. Appl. Microbiol. 2002, 92, 163s–170s. [Google Scholar] [CrossRef] [PubMed]
- Kulik, E.M.; Waltimo, T.; Weiger, R.; Schweizer, I.; Lenkeit, K.; Filipuzzi-Jenny, E.; Walter, C. Development of resistance of mutans streptococci and Porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin. Oral Investig. 2015, 19, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef] [Green Version]
- Moyed, H.S.; Broderick, S.H. Molecular cloning and expression of hipA, a gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1986, 166, 399–403. [Google Scholar] [CrossRef] [Green Version]
- Lafleur, M.D.; Qi, Q.; Lewis, K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhou, C.; Ren, B.; Li, X.; Weir, M.D.; Masri, R.M.; Oates, T.W.; Cheng, L.; Xu, H.K.H. Formation of persisters in Streptococcus mutans biofilms induced by antibacterial dental monomer. J. Mater. Sci. Mater. Med. 2017, 28, 178. [Google Scholar] [CrossRef]
- LaFleur, M.D.; Kumamoto, C.A.; Lewis, K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 2006, 50, 3839–3846. [Google Scholar] [CrossRef] [Green Version]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, W. Microbial persistence. Yale J. Biol. Med. 1958, 30, 257–291. [Google Scholar] [PubMed]
- Fauvart, M.; De Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Windels, E.M.; Michiels, J.E.; Van den Bergh, B.; Fauvart, M.; Michiels, J. Antibiotics: Combatting Tolerance to Stop Resistance. mBio 2019, 10, e02095-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, V.; Ajdic, D.; Koyanagi, S.; Levesque, C.M. The formation of Streptococcus mutans persisters induced by the quorum-sensing peptide pheromone is affected by the LexA regulator. J. Bacteriol. 2015, 197, 1083–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, e01162-16. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, Y.; Xu, C.; Zhang, X.; Li, J.; Dong, G.; Cao, J.; Zhou, T. Chlorhexidine exposure of clinical Klebsiella pneumoniae strains leads to acquired resistance to this disinfectant and to colistin. Int. J. Antimicrob. Agents 2019, 53, 864–867. [Google Scholar] [CrossRef]
- Amato, S.M.; Fazen, C.H.; Henry, T.C.; Mok, W.W.; Orman, M.A.; Sandvik, E.L.; Volzing, K.G.; Brynildsen, M.P. The role of metabolism in bacterial persistence. Front. Microbiol. 2014, 5, 70. [Google Scholar] [CrossRef] [Green Version]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Bottner, A.; He, R.Y.; Sarbu, A.; Nainar, S.M.H.; Dufour, D.; Gong, S.G.; Levesque, C.M. Streptococcus mutans isolated from children with severe-early childhood caries form higher levels of persisters. Arch. Oral Biol. 2019, 110, 104601. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Weiger, R.; Walter, C.; Zitzmann, N.U.; Waltimo, T. Microcalorimetric determination of the effects of amoxicillin, metronidazole, and their combination on in vitro biofilm. J. Periodontol. 2014, 85, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Spoering, A.L.; Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suppiger, S.; Astasov-Frauenhoffer, M.; Schweizer, I.; Waltimo, T.; Kulik, E.M. Tolerance and Persister Formation in Oral Streptococci. Antibiotics 2020, 9, 167. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040167
Suppiger S, Astasov-Frauenhoffer M, Schweizer I, Waltimo T, Kulik EM. Tolerance and Persister Formation in Oral Streptococci. Antibiotics. 2020; 9(4):167. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040167
Chicago/Turabian StyleSuppiger, Stephanie, Monika Astasov-Frauenhoffer, Irene Schweizer, Tuomas Waltimo, and Eva M. Kulik. 2020. "Tolerance and Persister Formation in Oral Streptococci" Antibiotics 9, no. 4: 167. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040167



