Synergy between Florfenicol and Aminoglycosides against Multidrug-Resistant Escherichia coli Isolates from Livestock
Abstract
:1. Introduction
2. Results and Discussion
2.1. Combination of Amphenicols and Aminoglycosides against MDR E. coli Isolates In Vitro
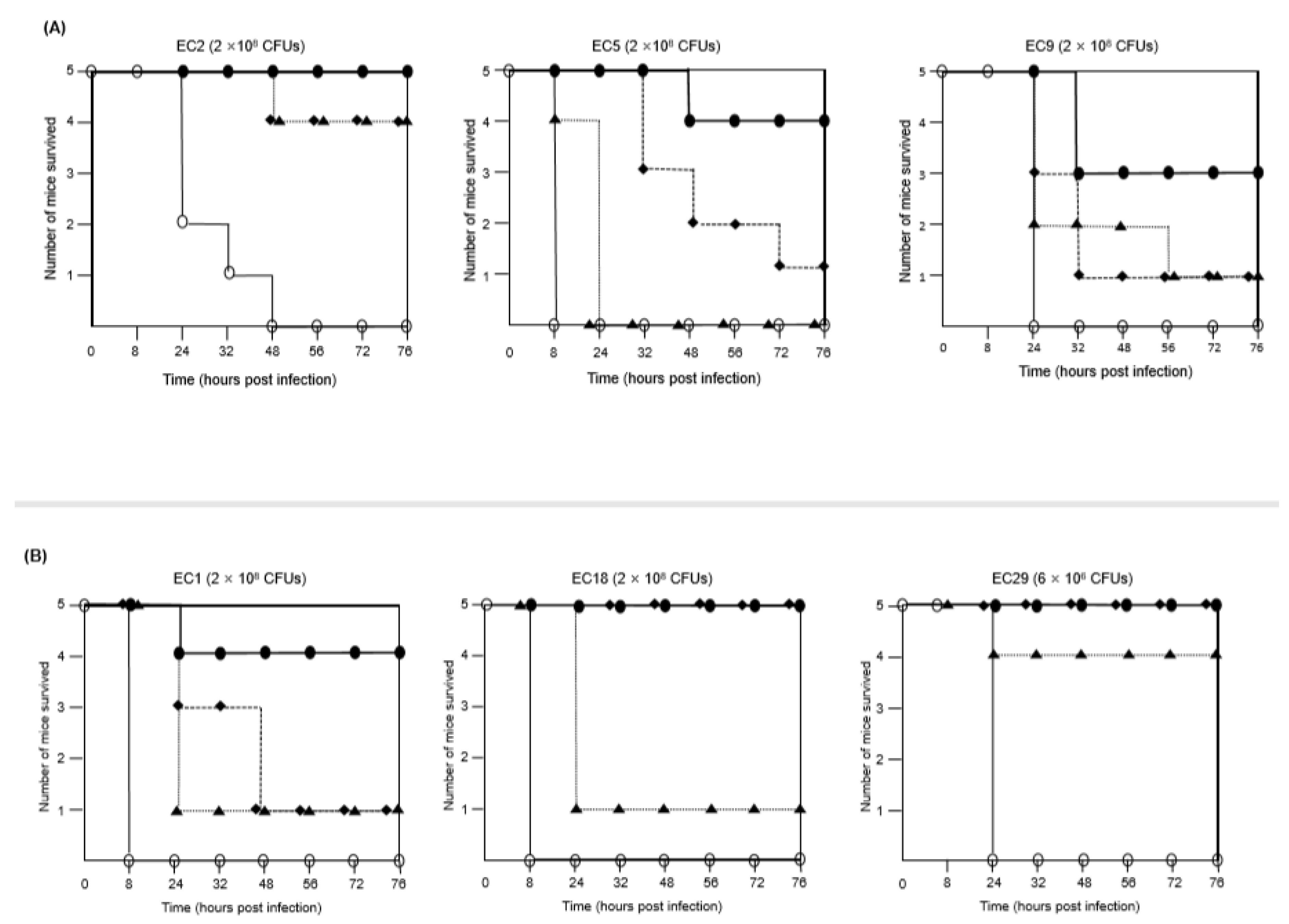
2.2. Combination of FFL and Aminoglycosides against MDR E. coli Isolates In Vivo
3. Materials and Methods
3.1. Bacterial Strains
3.2. Antimicrobial Susceptibility Testing
3.3. Polymerase Chain Reaction (PCR) Analysis of Aminoglycoside-Modifying Enzyme Genes
3.4. Checkerboard Assay
3.5. Mutation Frequency of E. coli Isolates
3.6. In Vivo Animal Experiments
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 289–316. [Google Scholar] [CrossRef] [Green Version]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Pogue, J.M.; Kaye, K.; Cohen, D.; Marchaim, D. Appropriate antimicrobial therapy in the era of multidrug-resistant human pathogens. Clin. Microbiol. Infect. 2015, 21, 302–312. [Google Scholar] [CrossRef] [Green Version]
- Cock, I.; Cheesman, M.J.; Ilanko, A.; Blonk, B. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokla, E.M.E.; Abutaleb, N.S.; Milik, S.; Li, D.; El-Baz, K.; Shalaby, M.-A.W.; Al-Karaki, R.; Nasr, M.; Klein, C.D.; Abouzid, K.A.; et al. Development of benzimidazole-based derivatives as antimicrobial agents and their synergistic effect with colistin against gram-negative bacteria. Eur. J. Med. Chem. 2020, 186, 111850. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Narasimhan, B. Antimicrobial Activity of Diazenyl Derivatives: An Update. Curr. Top. Med. Chem. 2018, 18, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Hagel, S.; Forstner, C. Who benefits from antimicrobial combination therapy? Lancet Infect. Dis. 2017, 17, 677–678. [Google Scholar] [CrossRef]
- A Hill, J.; Cowen, L.E. Using combination therapy to thwart drug resistance. Futur. Microbiol. 2015, 10, 1719–1726. [Google Scholar] [CrossRef]
- Davis, B.D. Bactericidal synergism between beta-lactams and aminoglycosides: Mechanism and possible therapeutic implications. Rev. Infect. Dis. 1982, 4, 237–245. [Google Scholar] [CrossRef]
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Assane, I.M.; Gozi, K.S.; Valladão, G.M.R.; Pilarski, F. Combination of antimicrobials as an approach to reduce their application in aquaculture: Emphasis on the use of thiamphenicol/florfenicol against Aeromonas hydrophila. Aquaculture 2019, 507, 238–245. [Google Scholar] [CrossRef]
- White, D.G.; Hudson, C.; Maurer, J.J.; Ayers, S.; Zhao, S.; Lee, M.D.; Bolton, L.; Foley, T.; Sherwood, J. Characterization of Chloramphenicol and Florfenicol Resistance in Escherichia coli Associated with Bovine Diarrhea. J. Clin. Microbiol. 2000, 38, 4593–4598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.S.G.; Nunes, B.; Almeida, J.; Guilhermino, L. Acute toxicity of oxytetracycline and florfenicol to the microalgae Tetraselmis chuii and to the crustacean Artemia parthenogenetica. Ecotoxicol. Environ. Saf. 2007, 67, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Animal and Plant Quarantine Agency (APQA). Antimicrobial Use and Antimicrobial Resistance Monitoring in Animals and Animal Products; APQA: Gimcheon, Korea, 2019.
- Dowling, P.M. Aminoglycosides. In Antimicrobial Therapy in Veterinary Medicine, 4th ed.; Giguère, S., Prescott, J.F., Baggot, J.D., Walker, R.D., Dowling, P.M., Eds.; Wiley Blackwell Publishing: Ames, IA, USA, 2017; pp. 207–229. [Google Scholar]
- Wei, C.-F.; Shien, J.-H.; Chang, S.-K.; Chou, C.-C. Florfenicol As a Modulator Enhancing Antimicrobial Activity: Example Using Combination with Thiamphenicol against Pasteurella multocida. Front. Microbiol. 2016, 7, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattanapanadda, P.; Kuo, H.-C.; Vickroy, T.W.; Sung, C.-H.; Rairat, T.; Lin, T.-L.; Yeh, S.-Y.; Chou, C.-C. In vitro and in vivo Synergistic Effects of Florfenicol and Thiamphenicol in Combination Against Swine Actinobacillus pleuropneumoniae and Pasteurella multocida. Front. Microbiol. 2019, 10, 2430. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-seventh Informational Supplement M100-S28; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Animal and Plant Quarantine Agency (APQA). National Antimicrobial Resistance Monitoring System in Korea; APQA: Gimcheon, Korea, 2014.
- Belaynehe, K.M.; Shin, S.W.; Hong-Tae, P.; Yoo, H.S. Occurrence of aminoglycoside-modifying enzymes among isolates of Escherichia coli exhibiting high levels of aminoglycoside resistance isolated from Korean cattle farms. FEMS Microbiol. Lett. 2017, 364, 129. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.M.; Fritsche, T.R.; Sader, H.S.; Jones, R.N. Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. Int. J. Antimicrob. Agents 2006, 27, 557–560. [Google Scholar] [CrossRef]
- Dawis, M.A.; Isenberg, H.D.; France, K.A.; Jenkins, S.G. In vitro activity of gatifloxacin alone and in combination with cefepime, meropenem, piperacillin and gentamicin against multidrug-resistant organisms. J. Antimicrob. Chemother. 2003, 51, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Yang, F.; Kong, T.; Wang, G.; Bai, D.; Liu, B. Pharmacokinetics of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus) at three temperatures after one single intramuscular injection. J. Veter- Pharmacol. Ther. 2018, 41, 739–745. [Google Scholar] [CrossRef]
- Varzi, H.N.; Esmailzadeh, S.; Morovvati, H.; Avizeh, R.; Shahriari, A.; Givi, M.E. Effect of silymarin and vitamin E on gentamicin-induced nephrotoxicity in dogs. J. Veter Pharmacol. Ther. 2007, 30, 477–481. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Singh, S.D.; Jayachandran, C. Effect of fever on pharmacokinetics and dosage regimen of intramuscularly administered amikacin in goats. J. Veter Sci. 2001, 2, 91–96. [Google Scholar] [CrossRef]
- Baggot, J.D.; Ling, G.V.; Chatfield, R.C. Clinical pharmacokinetics of amikacin in dogs. Am. J. Veter Res. 1985, 46, 1793–1796. [Google Scholar]
- Thadepalli, H.; Hajji, M.; Perumal, V.K.; Chuah, S.K.; Gollapudi, S. Evaluation of temafloxacin in a rat model of intra-abdominal abscess. J. Antimicrob. Chemother. 1992, 29, 687–692. [Google Scholar] [CrossRef] [PubMed]
| Isolate No. | Animals | Samples | Isolated Year | Resistance Pattern | Aminoglycoside-Modifying Enzyme Gene |
|---|---|---|---|---|---|
| EC1 | Pig | Feces | 2016 | STR, AMP, AMX, CEF, NAL, CIP, CHL, FFL, TET, SXT | aph(3”)-Ia, aph(3”)-Ib |
| EC2 | Pig | Feces | 2016 | GEN, AMK, STR, KAN, AMP, AMX, NAL, CIP, TET, SXT | aac(3)-IVa, ant(2”)-Ia, ant(3”)-Ia, aph(3’)-Ia |
| EC5 | Pig | Intestinal lesion | 2015 | COL, GEN, STR, KAN, AMP, AMX, TET, SXT | aac(3)-IVa, ant(3”)-Ia, aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC9 | Pig | Feces | 2016 | STR, KAN, AMP, AMX, NAL, CIP, CHL, FFL, TET | aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC14 | Chicken | Liver | 2016 | STR, AMP, AMX, TET, SXT | ant(3”)-Ia |
| EC15 | Chicken | Oviduct | 2016 | STR, KAN, AMP, AMX, AMC, CEF, NAL, CIP, CHL, TET, SXT | ant(3”)-Ia, aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC18 | Pig | Feces | 2016 | AMK, STR, KAN, AMP, AMX, CEF, NAL, CIP, CHL, FFL, TET, SXT | aac(6’)-Ib, ant(2”)-Ia, ant(3”)-Ia, aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC19 | Pig | Intestinal lesion | 2016 | GEN, STR, AMP, AMX, CHL, FFL, TET, SXT | aac(3)-IIa, ant(2”)-Ia, ant(3”)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC24 | Pig | Urinary tract | 2015 | AMK, STR, KAN, AMP, AMX, CEF, CHL, FFL, TET, SXT | ant(2”)-Ia, ant(3”)-Ia, aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC28 | Pig | Intestinal lesion | 2015 | COL, GEN, AMK, STR, KAN, AMP, AMX, CHL, FFL, TET | ant(2”)-Ia, ant(3”)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| EC29 | Pig | Feces | 2011 | COL, GEN, STR, KAN, AMP, AMX, NAL, CIP, CHL, FFL, TET, SXT | aac(3)-IVa, ant(2”)-Ia, ant(3”)-Ia, aph(3’)-Ia, aph(3”)-Ia, aph(3”)-Ib |
| Antimicrobial Combination | Number of Isolates (%) | |||
|---|---|---|---|---|
| Synergy (FICI ≤ 0.5) | Partial Synergy (0.5 < FICI < 1) | Additive (FICI = 1) | Indifference (1 < FICI < 4) | |
| CEF/GEN | 2 (18.2) | 2 (18.2) | 0 | 7 (63.6) |
| FFL/GEN | 6 (54.5) | 3 (27.3) | 0 | 2 (18.2) |
| FFL/AMK | 10 (90.9) | 1 (9.1) | 0 | 0 |
| CHL/GEN | 6 (54.5) | 3 (27.3) | 0 | 2 (18.2) |
| CHL/AMK | 8 (72.7) | 3 (27.3) | 0 | 0 |
| FFL/CEF | 0 | 3 (27.3) | 3 (27.3) | 5 (45.5) |
| E. coli Isolates | Frequency of Mutants Growing in Media Containing Antimicrobial Agents | ||
|---|---|---|---|
| EC10 (MIC of FFL, 4 μg/mL; MIC of GEN, 4 μg/mL) | FFL (32 μg/mL) | GEN (16 μg/mL) | FFL (32 μg/mL) and GEN (16 μg/mL) |
| 2.3 × 10−5 | 5.2 × 10−5 | 8 × 10−6 | |
| EC15 (MIC of FFL, 4 μg/mL; MIC of AMK, 8 μg/mL) | FFL (32 μg/mL) | AMK (64 μg/mL) | FFL (32 μg/mL) and AMK (64 μg/mL) |
| 2 × 10−7 | 1.4 × 10−6 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Woo, J.H.; Jun, S.H.; Moon, D.C.; Lim, S.-K.; Lee, J.C. Synergy between Florfenicol and Aminoglycosides against Multidrug-Resistant Escherichia coli Isolates from Livestock. Antibiotics 2020, 9, 185. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040185
Kim S, Woo JH, Jun SH, Moon DC, Lim S-K, Lee JC. Synergy between Florfenicol and Aminoglycosides against Multidrug-Resistant Escherichia coli Isolates from Livestock. Antibiotics. 2020; 9(4):185. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040185
Chicago/Turabian StyleKim, Shukho, Jung Hwa Woo, So Hyun Jun, Dong Chan Moon, Suk-Kyung Lim, and Je Chul Lee. 2020. "Synergy between Florfenicol and Aminoglycosides against Multidrug-Resistant Escherichia coli Isolates from Livestock" Antibiotics 9, no. 4: 185. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040185






