Management of a Case of Peritonitis Due to Neisseria gonorrhoeae Infection Following Pelvic Inflammatory Disease (PID)
Abstract
:1. Introduction
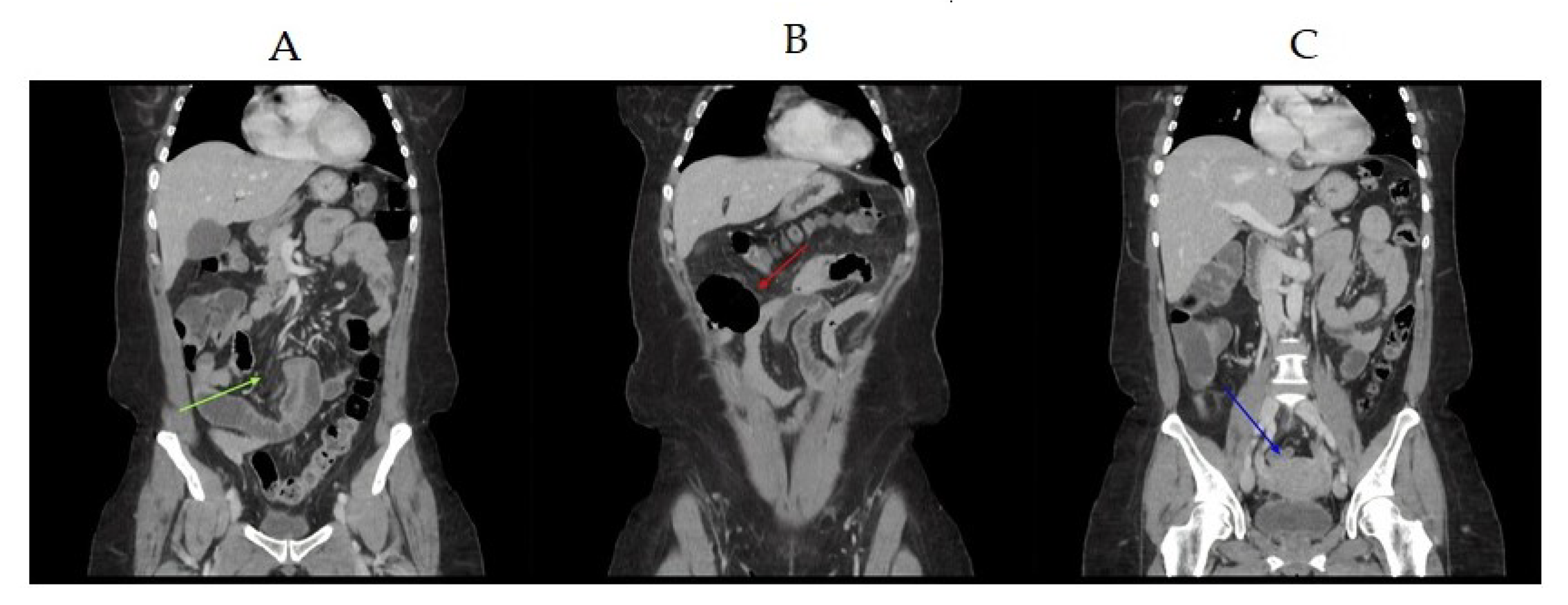
2. Case Study
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Soper, D.E. Pelvic inflammatory disease. Obstet. Gynecol. 2010, 116, 419–428. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sexually Transmitted Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 14 June 2019).
- Taylor, B.D.; Zheng, X.; O’Connell, C.M.; Wiesenfeld, H.C.; Hillier, S.L.; Darville, T. Risk factors for Mycoplasma genitalium endometritis and incident infection: A secondary data analysis of the T cell response against Chlamydia (TRAC) study. Sex Transm. Infect. 2018, 94, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, C.L.; Totten, P.A.; Tang, G.; Astete, S.G.; Ferris, M.J.; Norori, J.; Bass, D.C.; Martin, D.H.; Taylor, B.D.; Ness, R.B. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm. Infect. 2016, 92, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCormac, W.M. Pelvic inflammatory disease. N. Engl. J. Med. 1994, 330, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, G.; Cole, M.; Unemo, M.; Hoffmann, S.; Ison, C.; van de Laar, M. The European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP)-a sentinel approach in the European Union (EU)/European Economic Area (EEA). Sex Transm. Infect. 2013, 89, 16–18. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoints Tables for Interpretation of MICs and Zone Diameters, Version 9.0, 2019. EUCAST. Available online: http://www.eucast.org (accessed on 1 January 2020).
- Unemo, M.; Golparian, D.; Sánchez-Busó, L.; Grad, Y.; Jacobsson, S.; Ohnishi, M.; Lahra, M.M.; Limnios, A.; Sikora, A.E.; Wi, T.; et al. The novel 2016 WHO Neisseria gonorrhoeae reference strains for global quality assurance of laboratory investigations: Phenotypic, genetic and reference genome characterization. J. Antimicrob. Chemother. 2016, 71, 3096–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, I.M.; Ison, C.A.; Aanensen, D.M.; Fenton, K.A.; Spratt, B.G. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J. Infect. Dis. 2004, 189, 1497–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Molecular Typing of Neisseria gonorrhoeae-A Study of 2013 Isolates, 2018. Available online: https://ecdc.europa.eu/sites/portal/files/documents/Molecular-typing-N-gonorrhoeaeweb.pdf. (accessed on 30 May 2018).
- Tanaka, M.; Nakayama, H.; Haraoka, M.; Saika, T. Antimicrobial resistance of Neisseria gonorrhoeae and high prevalence of ciprofloxacin-resistant isolates in Japan, 1993 to 1998. J. Clin. Microbiol. 2000, 38, 521–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, N.G.; Clark, L.R.; Jaeger, J.R. Fitz-Hugh-Curtis syndrome: A diagnosis to consider in women with right upper quadrant pain. Cleve Clin. J. Med. 2004, 71, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Carannante, A.; Ciammaruconi, A.; Vacca, P.; Anselmo, A.; Fillo, S.; Palozzi, A.M.; Fortunato, A.; Lista, F.; Stefanelli, P. Genomic Characterization of Gonococci from Different Anatomic Sites, Italy, 2007–2014. Microb. Drug Resist. 2019, 25, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Brunel, A.S.; Fraisse, T.; Lechiche, C.; Sotto, A.; Laporte, S. A sexually transmitted peritonitis. Med. Mal. Infect. 2008, 38, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, W.; Dhar, R. Unexplained peritonitis due to Neisseria gonorrhoeae secondary to a sterile tubo-ovarian abscess. Kuwait Med. J. 2015, 47, 166–167. [Google Scholar]
- Wilmore, S.M.S.; Reynolds, C.J. Gonococcal peritonitis diagnosed post laparotomy in a 38-year-old woman: A case report. Cases J. 2009, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Antimicrobial | MIC Value (mg/L) |
|---|---|
| Cefixime | 0.047 |
| Ceftriaxone | 0.016 |
| Ciprofloxacin | 12 |
| Azithromycin | 1 |
| Spectinomycin | 12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francesco, M.A.D.; Stefanelli, P.; Carannante, A.; Corbellini, S.; Giagulli, C.; Lorenzin, G.; Ronconi, M.; Arici, E.; Cadei, M.; Campora, R.; et al. Management of a Case of Peritonitis Due to Neisseria gonorrhoeae Infection Following Pelvic Inflammatory Disease (PID). Antibiotics 2020, 9, 193. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040193
Francesco MAD, Stefanelli P, Carannante A, Corbellini S, Giagulli C, Lorenzin G, Ronconi M, Arici E, Cadei M, Campora R, et al. Management of a Case of Peritonitis Due to Neisseria gonorrhoeae Infection Following Pelvic Inflammatory Disease (PID). Antibiotics. 2020; 9(4):193. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040193
Chicago/Turabian StyleFrancesco, Maria A. De, Paola Stefanelli, Anna Carannante, Silvia Corbellini, Cinzia Giagulli, Giovanni Lorenzin, Maurizio Ronconi, Elisa Arici, Monica Cadei, Riccardo Campora, and et al. 2020. "Management of a Case of Peritonitis Due to Neisseria gonorrhoeae Infection Following Pelvic Inflammatory Disease (PID)" Antibiotics 9, no. 4: 193. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040193






