Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin
Abstract
:1. Introduction
2. Results
2.1. Pharmacokinetic Analysis of Macrolides
2.2. Distribution of Macrolides to Tissues and ISF
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pharmacokinetic Study of Macrolides
4.3. Tissue Distribution of Macrolides
4.4. Distribution of Macrolides to ISF
4.5. Macrolides Assay
4.6. Pharmacokinetic Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roope, L.S.J.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V.; et al. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, 6435. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.R. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 2001, 7, 589–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Homma, T.; Hori, T.; Ohshiro, M.; Maki, H.; Yamano, Y.; Shimada, J.; Kuwahara, S. In vitro pharmacokinetic and pharmacodynamic evaluation of S-013420 against Haemophilus influenzae and Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2010, 54, 4300–4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, tissue, and intracellular concentrations of azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 2013, 57, 1736–1742. [Google Scholar] [CrossRef] [Green Version]
- De La Peña, A.; Dalla Costa, T.; Talton, J.D.; Rehak, E.; Gross, J.; Thyroff-Friesinger, U.; Webb, A.I.; Müller, M.; Derendorf, H. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm. Res. 2001, 18, 1310–1314. [Google Scholar] [CrossRef]
- Togami, K.; Chono, S.; Morimoto, K. Distribution characteristics of clarithromycin and azithromycin, macrolide antimicrobial agents used for treatment of respiratory infections, in lung epithelial lining fluid and alveolar macrophages. Biopharm. Drug Dispos. 2011, 32, 389–397. [Google Scholar] [CrossRef]
- Togami, K.; Chono, S.; Morimoto, K. Subcellular distribution of azithromycin and clarithromycin in rat alveolar macrophages (NR8383) in vitro. Biol. Pharm. Bull. 2013, 36, 1494–1499. [Google Scholar] [CrossRef] [Green Version]
- Danesi, R.; Lupetti, A.; Barbara, C.; Ghelardi, E.; Chella, A.; Malizia, T.; Senesi, S.; Angeletti, C.A.; Del Tacca, M.; Campa, M. Comparative distribution of azithromycin in lung tissue of patients given oral daily doses of 500 and 1000 mg. J. Antimicrob. Chemother. 2003, 51, 939–945. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Matzneller, P.; Zeitlinger, M.; Schmidt, S. Development of a population pharmacokinetic model characterizing the tissue distribution of azithromycin in healthy subjects. Antimicrob. Agents Chemother. 2014, 58, 6675–6684. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Inagaki, Y.; Kobuchi, S.; Takada, K.; Sakaeda, T. Therapeutic drug monitoring of vancomycin in dermal interstitial fluid using dissolving microneedles. Int. J. Med. Sci. 2016, 13, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Taniguchi, M.; Hayashi, A.; Anai, M.; Morita, S.; Ko, E.; Yoshimoto, N.; Yoshii, Y.; Kobuchi, S.; Sakaeda, T.; et al. Application of dissolving microneedles to glucose monitoring through dermal interstitial fluid. Biol. Pharm. Bull. 2014, 37, 1776–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobuchi, S.; Aoki, M.; Inoue, C.; Murakami, H.; Kuwahara, A.; Nakamura, T.; Yasui, H.; Ito, Y.; Takada, K.; Sakaeda, T. Transport of Azithromycin into Extravascular Space in Rats. Antimicrob. Agents Chemother. 2016, 60, 6823–6827. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.J.; Pruul, H. Phagocyte uptake and transport of azithromycin. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Hand, W.L.; Hand, D.L. Characteristics and mechanisms of azithromycin accumulation and efflux in human polymorphonuclear leukocytes. Int. J. Antimicrob. Agents 2001, 18, 419–425. [Google Scholar] [CrossRef]
- Carlier, M.B.; Garcia-Luque, I.; Montenez, J.P.; Tulkens, P.M.; Piret, J. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int. J. Tissue. React. 1994, 16, 211–220. [Google Scholar] [PubMed]
- Mandell, G.L.; Coleman, E. Uptake, transport, and delivery of antimicrobial agents by human polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 2001, 45, 1794–1798. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, S.; Tulkens, P.M.; Van Bambeke, F. Cellular pharmacokinetics of the novel biaryloxazolidinone radezolid in phagocytic cells: Studies with macrophages and polymorphonuclear neutrophils. Antimicrob. Agents Chemother. 2010, 54, 2540–2548. [Google Scholar] [CrossRef] [Green Version]
- Pfizer Japan Inc. ZITHROMAC® [Drug Information]; Pfizer Japan Inc.: Tokyo, Japan, 2018. [Google Scholar]
- Molinari, G.; Rohde, M.; Guzmán, C.A.; Chhatwal, G.S. Two distinct pathways for the invasion of Streptococcus pyogenes in non-phagocytic cells. Cell Microbiol. 2000, 2, 145–154. [Google Scholar] [CrossRef]
- Nakagawa, I.; Nakata, M.; Kawabata, S.; Hamada, S. Cytochrome c-mediated caspase-9 activation triggers apoptosis in Streptococcus pyogenes-infected epithelial cells. Cell Microbiol. 2001, 3, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Liang, Z.; Mo, Z.; Zhang, J. The species distribution, antimicrobial resistance and risk factors for poor outcome of coagulase-negative staphylococci bacteraemia in China. Antimicrob. Resist. Infect. Control 2019, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Kohno, S.; Tateda, K.; Kadota, J.; Fujita, J.; Niki, Y.; Watanabe, A.; Nagashima, M. Contradiction between in vitro and clinical outcome: Intravenous followed by oral azithromycin therapy demonstrated clinical efficacy in macrolide-resistant pneumococcal pneumonia. J. Infect. Chemother. 2013, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Bishai, W. The in vivo-in vitro paradox in pneumococcal respiratory tract infections. J. Antimicrob. Chemother. 2002, 49, 433–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, C.D.; Nightingale, C.H.; Nicolau, D.P.; Belliveau, P.P.; Banevicius, M.A.; Quintiliani, R. Intracellular and extracellular penetration of azithromycin into inflammatory and noninflammatory blister fluid. Antimicrob. Agents Chemother. 1994, 38, 2449–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, A.; Scaglione, F.; Derendorf, H. Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. Int. J. Antimicrob. Agents 2010, 35, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Punnia-Moorthy, A. Evaluation of pH changes in inflammation of the subcutaneous air pouch lining in the rat, induced by carrageenan, dextran and Staphylococcus aureus. J. Oral Pathol. 1987, 16, 36–44. [Google Scholar] [CrossRef]
- Lee, A.K.; Lee, J.H.; Kwon, J.W.; Kim, W.B.; Kim, S.G.; Kim, S.H.; Lee, M.G. Pharmacokinetics of clarithromycin in rats with acute renal failure induced by uranyl nitrate. Biopharm. Drug Dispos. 2004, 25, 273–282. [Google Scholar] [CrossRef]
- Hanada, E.; Ohtani, H.; Kotaki, H.; Sawada, Y.; Sato, H.; Iga, T. Pharmacodynamic analysis of the electrocardiographic interaction between disopyramide and erythromycin in rats. J. Pharm. Sci. 1999, 88, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kobuchi, S.; Fujita, A.; Kato, A.; Kobayashi, H.; Ito, Y.; Sakaeda, T. Pharmacokinetics and lung distribution of macrolide antibiotics in sepsis model rats. Xenobiotica 2020, 50, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Filist, M.; Buś-Kwaśnik, K.; Ksycińska, H.; Rudzki, P.J. Simplified LC-MS/MS method enabling the determination of azithromycin in human plasma after a low 100 mg dose administration. J. Pharm. Biomed. Anal. 2014, 100, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Krizman-Matasic, I.; Terzic, S.; Ahel, M. Comprehensive determination of macrolide antibiotics, their synthesis intermediates and transformation products in wastewater effluents and ambient waters by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1509, 60–68. [Google Scholar] [CrossRef]
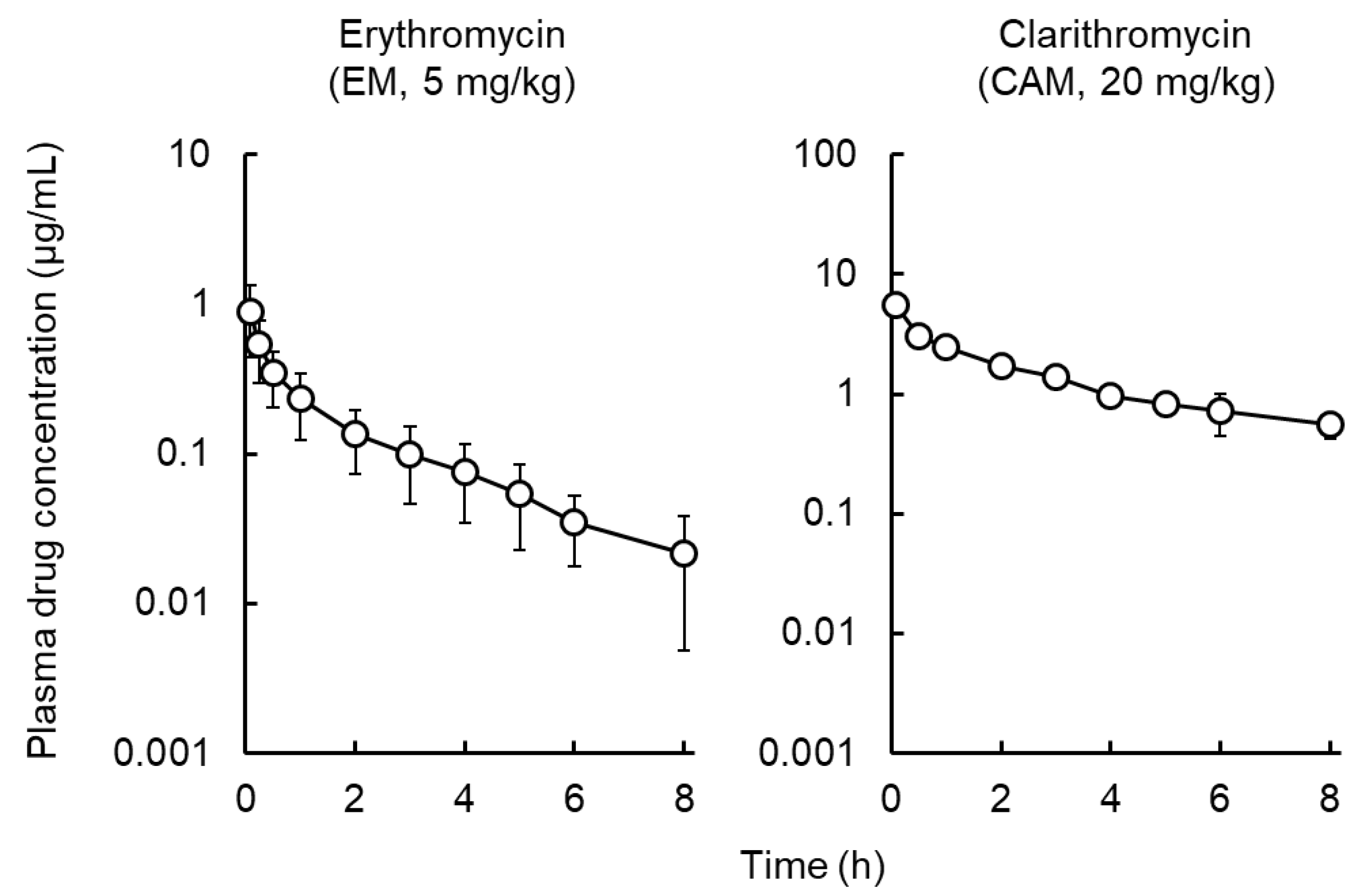
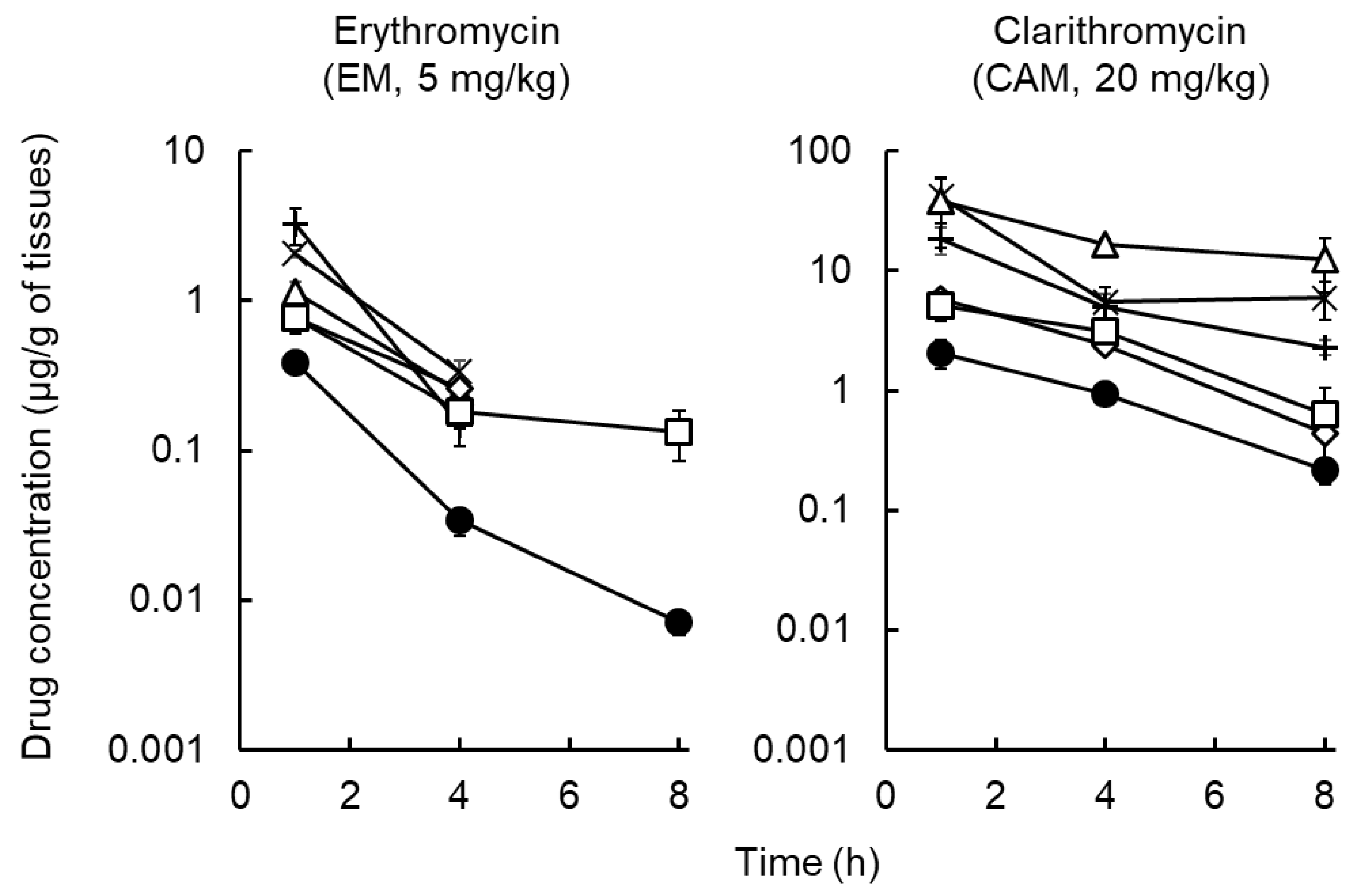
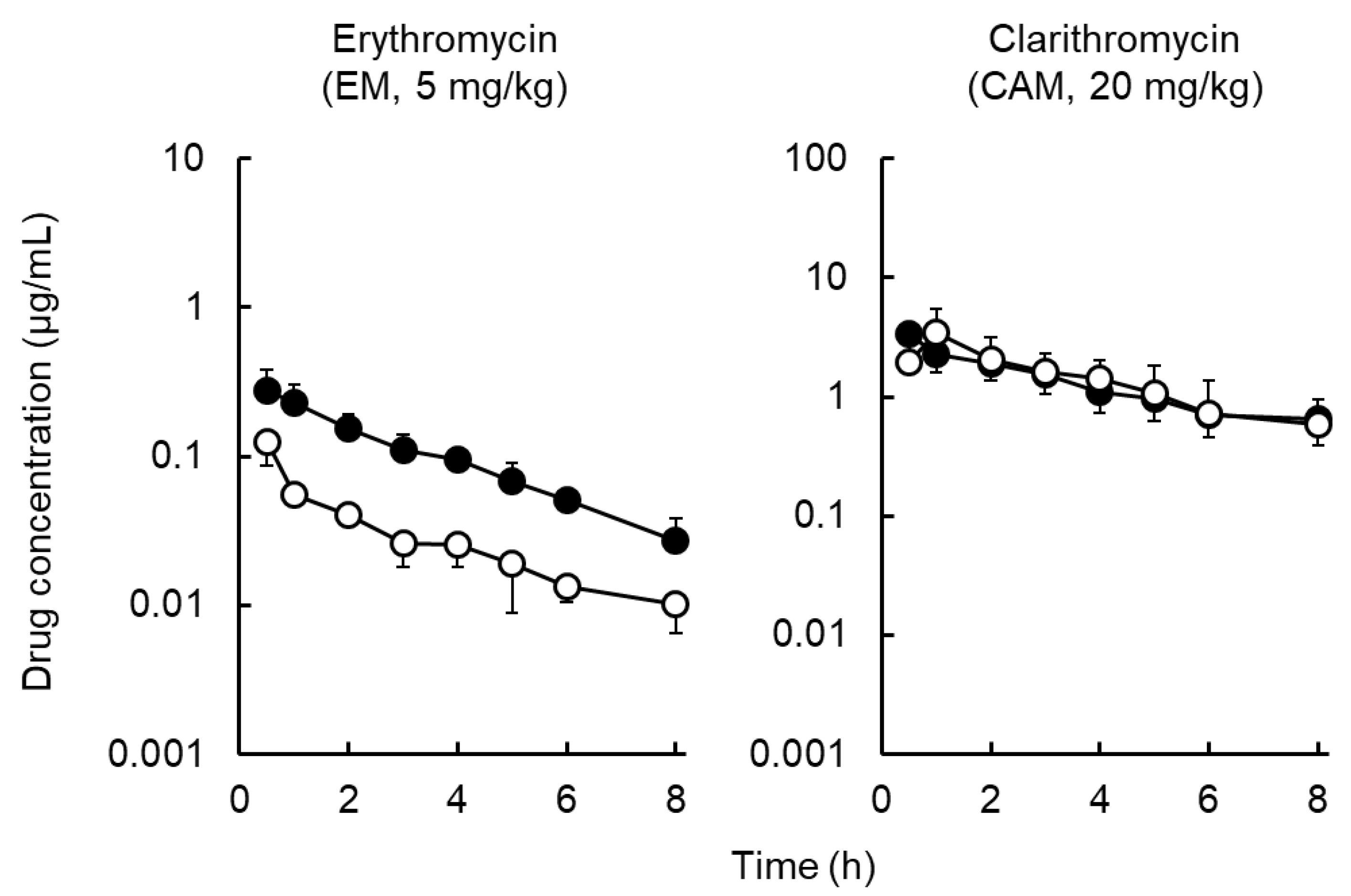
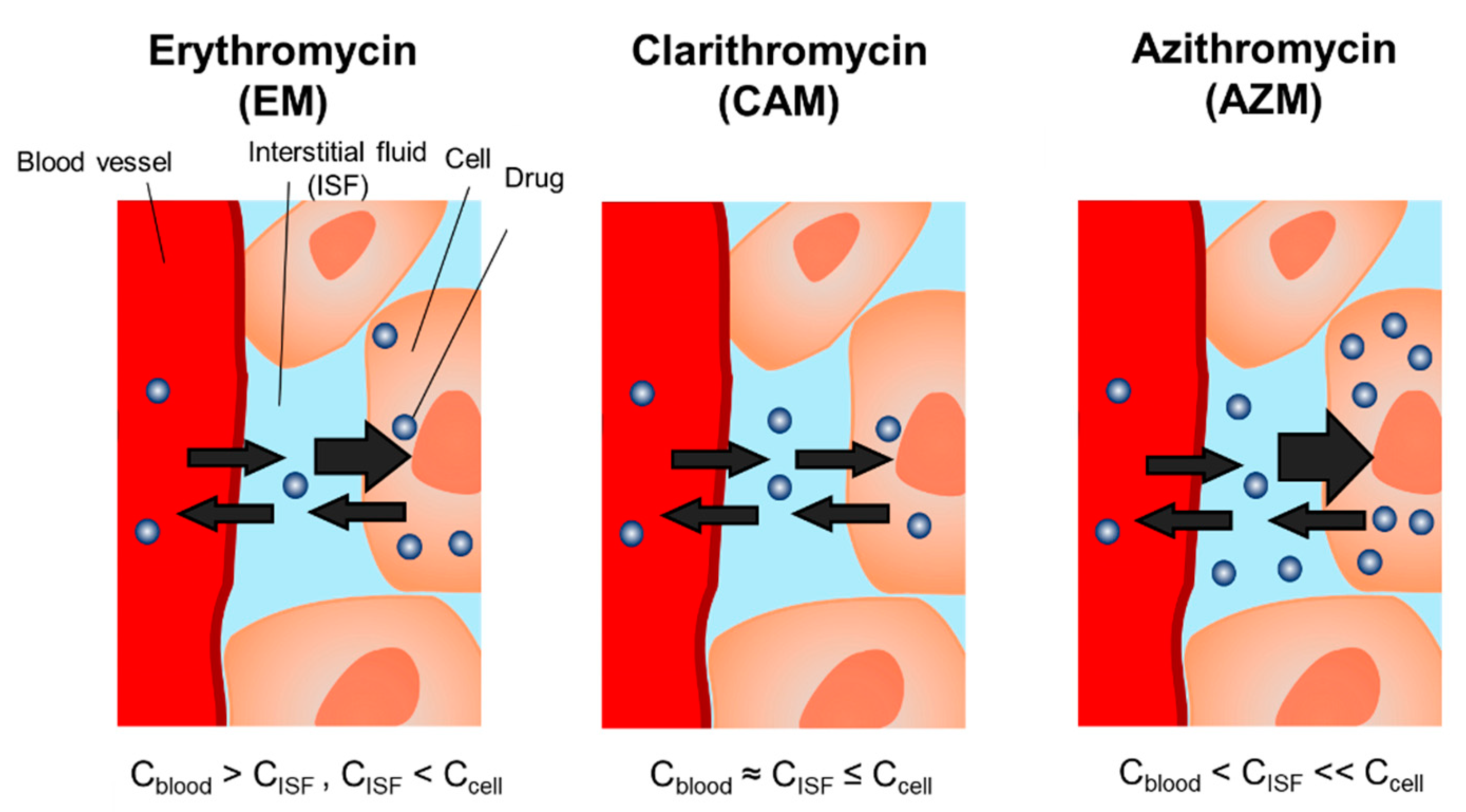
| Pharmacokinetic Parameters | Erythromycin (EM) | Clarithromycin (CAM) | Azithromycin a) (AZM) |
|---|---|---|---|
| t1/2, z (h) | 1.8 ± 0.4 | 3.3 ± 0.8 | 53.5 ± 32.6 |
| AUC0–∞ (μg∙h/mL) | 1.1 ± 0.5 | 13.8 ± 2.6 | 10.2 ± 4.1 |
| CLtot (L/h/kg) | 5.6 ± 2.6 | 1.5 ± 0.3 | 1.2 ± 0.7 |
| Vd (L/kg) | 13.8 ± 5.1 | 6.8 ± 0.8 | 68.5 ± 30.2 |
| MRT (h) | 2.2 ± 0.5 | 4.3 ± 1.0 | 64.9 ± 47.0 |
| Time (h) | Erythromycin (EM) | Clarithromycin (CAM) | Azithromycin a) (AZM) | |||
|---|---|---|---|---|---|---|
| ISF/Plasma | Skin/Plasma | ISF/Plasma | Skin/Plasma | ISF/Plasma | Skin/Plasma | |
| 1 | 0.27 ± 0.10 | 3.2 ± 0.9 | 1.5 ± 0.9 | 2.5 ± 0.4 | 3.8 ± 1.6 | 4.3 ± 1.5 |
| 4 | 0.27 ± 0.05 | 6.3 ± 2.3 | 1.5 ± 0.7 | 3.4 ± 0.4 | 5.0 ± 1.3 | 36.3 ± 23.8 |
| 8 | 0.39 ± 0.08 | 11.9 ± 3.8 *, # | 1.2 ± 0.9 | 2.9 ± 1.8 | – | – |
| 72 | – | – | – | – | 4.9 ± 0.8 | 83.1 ± 76.2 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobuchi, S.; Kabata, T.; Maeda, K.; Ito, Y.; Sakaeda, T. Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin. Antibiotics 2020, 9, 199. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040199
Kobuchi S, Kabata T, Maeda K, Ito Y, Sakaeda T. Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin. Antibiotics. 2020; 9(4):199. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040199
Chicago/Turabian StyleKobuchi, Shinji, Teruhiko Kabata, Koki Maeda, Yukako Ito, and Toshiyuki Sakaeda. 2020. "Pharmacokinetics of Macrolide Antibiotics and Transport into the Interstitial Fluid: Comparison among Erythromycin, Clarithromycin, and Azithromycin" Antibiotics 9, no. 4: 199. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9040199





