Self-Reported Antimicrobial Stewardship Practices in Primary Care Using the TARGET Antibiotics Self-Assessment Tool
Abstract
:1. Introduction
2. Results
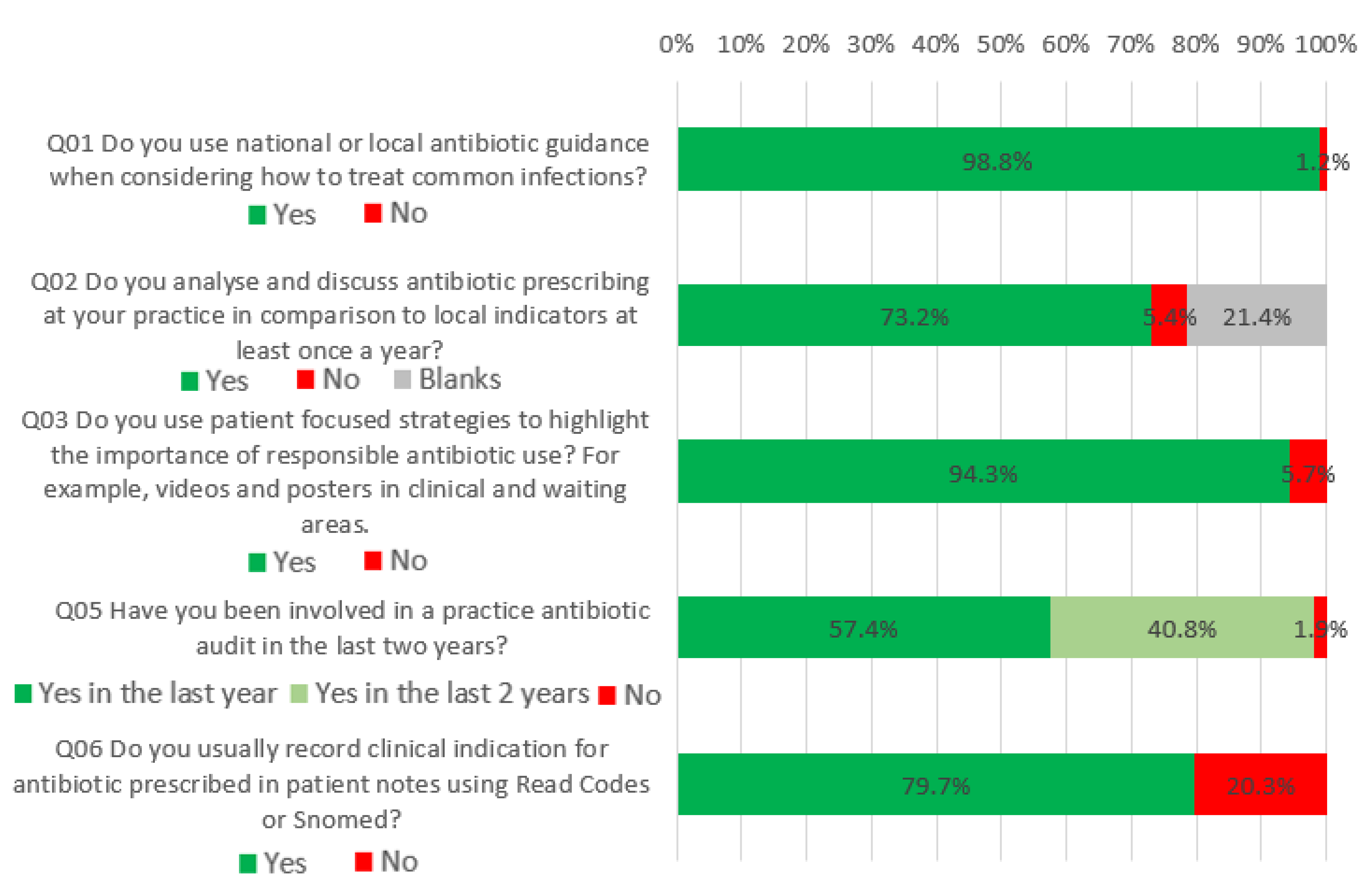
2.1. What Would be Good Practice Now
2.2. What Most Practices Should Aim to Do Soon
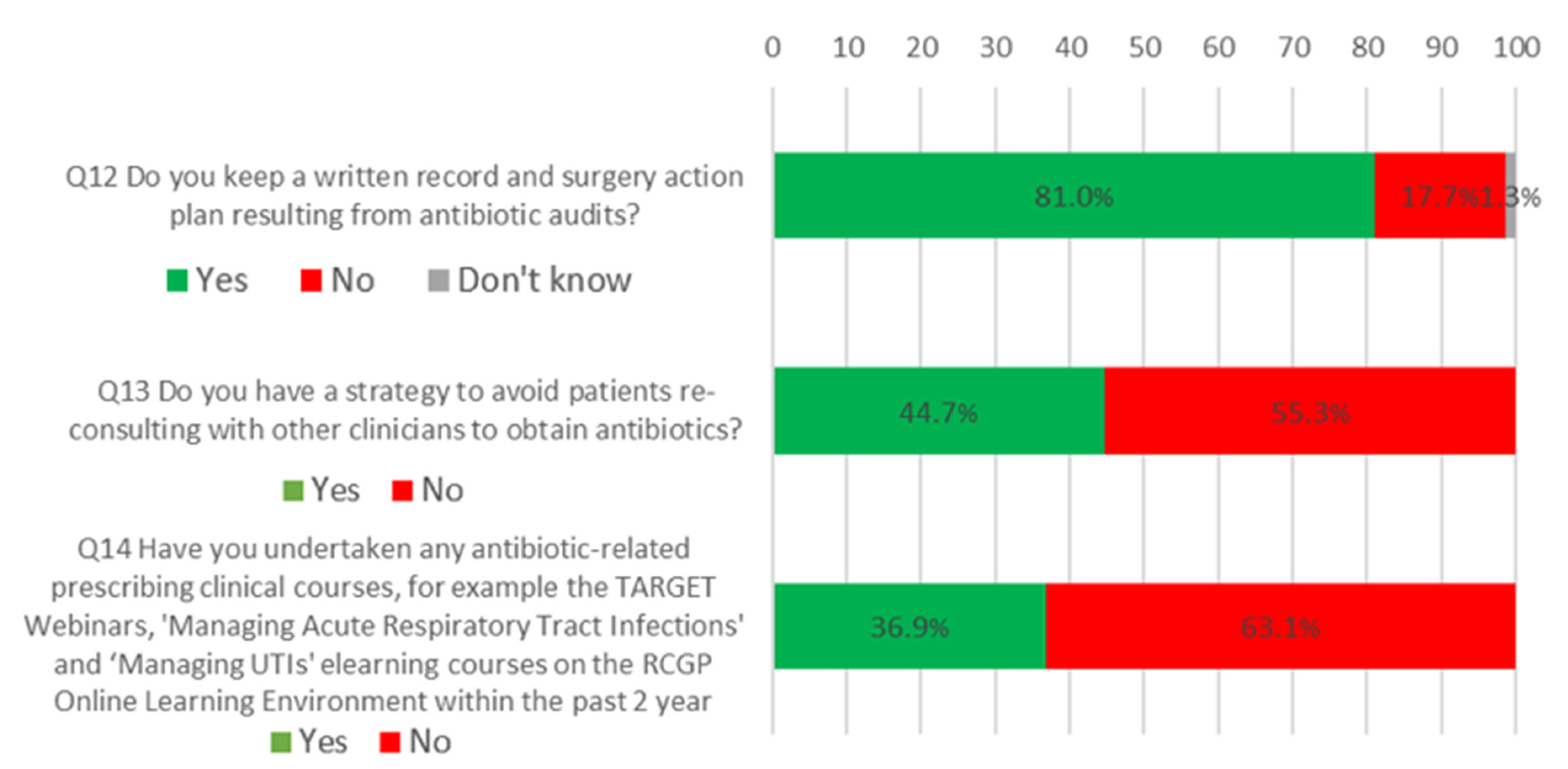
2.3. What All Antibiotic-Aware Practices Should Be Doing
2.4. Comparison with 2014–2016 Data
3. Discussion
3.1. Summary of the Findings
3.2. Comparison with Existing Literature
3.3. Strengths and Limitations
3.4. Implications for Research and Practice
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Question | Variable | Categories | Question Response | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Do you use antibiotic guidance | Workplace | GP practice Hospital OOH Dental practice Other | 23 1 0 0 4 | 2132 55 58 1 98 | 1.00 0.70 n.e. n.e. 0.33 | 0.08, 6.27 n.e. n.e. 0.08, 1.27 | 0.15 |
| Profession | GP Nurse Pharmacist Other | 21 2 3 2 | 1870 265 75 132 | 1.00 2.07 0.42 1.08 | 0.43, 9.95 0.10, 1.82 0.21, 5.48 | 0.3 | |
| Do you discuss antibiotic prescribing | Workplace | GP practice Hospital OOH Dental practice Other | 116 3 4 0 5 | 1592 43 40 0 60 | 1.00 0.96 0.70 n.e. 0.55 | 0.26, 3.59 0.23, 2.13 n.e. 0.19, 1.60 | 0.7 |
| Profession | GP Nurse Pharmacist Other | 115 2 2 9 | 1387 203 53 90 | 1.00 9.95 2.70 0.91 | 2.34, 42.3 0.61, 12.0 0.41, 2.00 | <0.001 | |
| Do you use patient focused strategies | Workplace | GP practice Hospital OOH Dental practice Other | 90 17 7 0 20 | 2065 39 51 1 82 | 1.00 0.21 0.38 n.e. 0.35 | 0.10, 0.43 0.16, 0.91 n.e. 0.19, 0.66 | <0.001 |
| Profession | GP Nurse Pharmacist Other | 65 29 13 27 | 1826 238 65 107 | 1.00 0.38 0.25 0.23 | 0.23, 0.62 0.12, 0.52 0.13, 0.40 | <0.001 | |
| Do you usually record clinical indication for antibiotic prescribed | Workplace | GP practice Hospital OOH Dental practice Other | 410 12 21 0 38 | 1745 44 37 1 64 | 1.00 0.93 0.37 n.e. 0.28 | 0.39, 2.20 0.16, 0.83 n.e. 0.13, 0.62 | <0.001 |
| Profession | GP Nurse Pharmacist Other | 381 51 13 36 | 1510 216 65 98 | 1.00 1.39 1.85 0.81 | 0.89, 2.19 0.79, 4.33 0.47, 1.40 | 0.14 | |
| Is the latest antibiotic guidance made available | Workplace | GP practice Hospital OOH Dental practice Other | 477 9 10 0 16 | 1225 26 18 0 38 | 1.00 0.99 0.63 n.e. 0.66 | 0.35, 2.85 0.21, 1.84 n.e. 0.28, 1.53 | 0.7 |
| Profession | GP Nurse Pharmacist Other | 453 38 13 8 | 1056 149 40 60 | 1.00 2.39 1.80 4.27 | 1.32, 4.34 0.77, 4.23 1.49, 12.2 | <0.001 | |
| Do you keep a written record | Workplace | GP practice Hospital OOH Dental practice Other | 356 16 15 0 32 | 1773 37 41 1 70 | 1.00 0.51 0.61 n.e. 0.43 | 0.26, 1.01 0.32, 1.15 n.e. 0.26, 0.73 | 0.001 |
| Profession | GP Nurse Pharmacist Other | 336 36 13 34 | 1535 228 65 92 | 1.00 1.72 1.61 0.77 | 1.14, 2.60 0.82, 3.16 0.48, 1.23 | 0.01 | |
| Do you have a strategy to avoid patient reconsulting | Workplace | GP practice Hospital OOH Dental practice Other | 1167 38 39 1 66 | 988 18 19 0 36 | 1.00 0.39 0.56 n.e. 0.48 | 0.14, 1.07 0.23, 1.40 n.e. 0.22, 1.04 | 0.08 |
| Profession | GP Nurse Pharmacist Other | 1057 146 40 68 | 834 121 38 66 | 1.00 1.22 1.85 1.94 | 0.77, 1.92 0.84, 4.10 1.03, 3.66 | 0.09 | |
| Have you undertaken any antibiotic-related courses | Workplace | GP practice Hospital OOH Dental practice Other | 1357 38 29 1 73 | 798 18 29 0 29 | 1.00 1.01 2.32 n.e. 0.69 | 0.45, 2.25 1.11, 4.86 n.e. 0.37, 1.30 | 0.09 |
| Profession | GP Nurse Pharmacist Other | 1167 183 52 96 | 724 84 26 38 | 1.00 0.72 0.92 0.62 | 0.48, 1.07 0.48, 1.77 0.36, 1.08 | 0.15 | |
| Variable | Categories | Question Response | p-Value | |||
|---|---|---|---|---|---|---|
| Yes in Last 2 Years | No | |||||
| Coeff. | 95% CI | Coeff. | 95% CI | |||
| Workplace | GP practice Hospital OOH Dental practice Other | 0.00 0.20 1.35 19.2 0.94 | −0.67, 1.08 0.45, 2.24 n.e. 0.22, 1.66 | 0.00 0.20 0.78 −1.01 −0.09 | −1.52, 1.93 −1.38, 2.94 n.e. −2.23, 2.05 | 0.01 |
| Profession | GP Nurse Pharmacist Other | 0.00 0.74 −0.41 1.90 | 0.28, 1.20 −1.13, 0.30 1.09, 2.71 | 0.00 0.09 −19.2 2.63 | −1.17, 1.35 n.e. 1.49, 3.77 | <0.001 |
| Variable | Categories | Question Response | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes, Computer | No | Other | |||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | ||||
| Workplace | GP practice Hospital OOH Dental practice Other | 0.00 0.35 −19.4 −19.6 −19.3 | −1.32, 2.03 n.e. n.e. n.e. | 0.00 −0.91 −0.82 −19.4 −1.37 | −1.68, −0.14 −1.51, −0.13 n.e. −2.06, −0.69 | 0.00 2.08 2.21 −1.57 −18.0 | −0.73, 4.88 −0.13, 4.56 n.e. n.e. | <0.001 | |
| Profession | GP Nurse Pharmacist Other | 0.00 0.30 −17.8 0.13 | −0.82, 1.41 n.e. −1.23, 1.49 | 0.00 0.11 1.00 −0.48 | −0.25, 0.48 0.26, 1.74 −0.97, 0.02 | 0.00 −17.7 −17.0 0.19 | n.e. n.e. −2.51, 2.90 | 0.02 | |
| Variable | Categories | Question Response | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Monthly | Yearly | No or Don’t Agree | ||||||
| Coeff. | 95% CI | Coeff. | 95% CI | Coeff. | 95% CI | |||
| Workplace | GP practice Hospital OOH Dental practice Other | 0.00 0.41 0.75 0.46 0.99 | −0.54, 1.36 0.01, 1.49 n.e. 0.32, 1.66 | 0.00 0.59 1.09 22.5 1.15 | −0.10, 1.28 0.45, 1.74 n.e. 0.63, 1.68 | 0.00 3.12 −18.3 18.6 2.69 | 1.05, 5.18 n.e. n.e. 0.31, 5.06 | <0.001 |
| Profession | GP Nurse Pharmacist Other | 0.00 −0.54 −0.71 −0.51 | −1.04, −0.04 −1.62, 0.19 −1.29, 0.27 | 0.00 0.48 0.55 1.60 | 0.11, 0.86 −0.05, 1.15 1.17, 2.03 | 0.00 −18.2 −18.3 0.78 | n.e. n.e. −1.24, 2.79 | <0.001 |
References
- Public Health England the TARGET Antibiotics Toolkit. Available online: http://www.rcgp.org.uk/targetantibiotics (accessed on 22 October 2018).
- Boud, D. Avoiding the traps: Seeking good practice in the use of self assessment and reflection in professional courses. Soc. Work Educ. 1999, 18, 121–132. [Google Scholar] [CrossRef]
- McMillan, J.H.; Hearn, J. Student self-assessment: The key to stronger student motivation and higher achievement. Educ. Horiz. 2008, 87, 40–49. [Google Scholar]
- Williams, B. Developing critical reflection for professional practice through problem-based learning. J. Adv. Nurs. 2001, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Royal College of General Practitioners Continuing Professional Development. Available online: https://www.rcgp.org.uk/training-exams/practice/revalidation/mythbusters-appraisal-and-revalidation/continuing-professional-development.aspx (accessed on 7 February 2020).
- Owens, R.; Jones, L.F.; Moore, M.; Pilat, D.; McNulty, C. Self-Assessment of Antimicrobial Stewardship in Primary Care: Self-Reported Practice Using the TARGET Primary Care Self-Assessment Tool. Antibiotics 2017, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.; Lecky, D.; Beech, E.; Ashiru-Oredope, D.; Costelloe, C.; Owens, R.; McNulty, C.A.M. Local implementation of national guidance on management of common infections in primary care in England: Findings of a mixed-methods national questionnaire. Pharm. J. 2020. [Google Scholar] [CrossRef] [Green Version]
- Allison, R.; Lecky, D.; Beech, E.; Ashiru-Oredope, D.; Costelloe, C.; Owens, R.; McNulty, C. What resources do NHS commissioning organisations use to support antimicrobial stewardship in primary care in England? Antibiotics 2020, 9, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Health and Care Excellence. Antimicrobial Stewardship: Changing Risk-related Behaviours in the General Population; National Institute for Health and Care Excellence: London, UK, 2017; Available online: https://www.nice.org.uk/guidance/ng63 (accessed on 7 February 2020).
- Public Health England. Summary of Antimicrobial Prescribing Guidance–Managing Common Infections; Public Health England: London, UK, 2020.
- Public Health England. Management of Infection Guidance for Primary Care for Consultation and Local Adaptation; Public Health England: London, UK, 2017.
- National Institute for Health and Care Excellence. Urinary Tract Infections in Adults; National Institute for Health and Care Excellence: London, UK, 2015. [Google Scholar]
- National Institute for Health and Care Excellence. Respiritory Tract Infections (Self-Limitting): Prescribing Antibiotics; National Institute for Health and Care Excellence: London, UK, 2008. [Google Scholar]
- Ivers, N.; Jamtvedt, G.; Flottorp, S.; Young, J.M.; Odgaard-Jensen, J.; French, S.D.; O’Brien, M.A.; Johansen, M.; Grimshaw, J.; Oxman, A.D. Audit and feedback: Effects on professional practice and healthcare outcomes. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.; Lecky, D.; Beech, E.; Costelloe, C.; Ashiru-Oredope, D.; Owens, R.; McNulty, C. What antimicrobial stewardship strategies do NHS commissioning organisations implement in primary care in England? J. Antimicrob. Chemother. 2020, in press. [Google Scholar]
- Colledge, A.; Car, J.; Donnelly, A.; Majeed, A. Health information for patients: Time to look beyond patient information leaflets. J. R. Soc. Med. 2008, 101, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadoorian, J.; Batty, H.P. An evidence-based model of effective self-assessment for directing professional learning. J. Dent. Educ. 2005, 69, 1315–1323. [Google Scholar] [PubMed]
- Austin, Z.; Gregory, P.A.M. Evaluating the accuracy of pharmacy students’ self-assessment skills. Am. J. Pharm. Educ. 2007, 71, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, P.; Norman, G. Self-assessment or self deception? A lack of association between nursing students’ self-assessment and performance. J. Adv. Nurs. 2011, 67, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Mazmanian, P.E.; Fordis, M.; Van Harrison, R.; Thorpe, K.E.; Perrier, L. Accuracy of physician self-assessment compared with observed measures of competence: A systematic review. J. Am. Med. Assoc. 2006, 296, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J. Lake Wobegon be gone! The “below-average effect” and the egocentric nature of comparative ability judgments. J. Pers. Soc. Psychol. 1999, 77, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Jenner, E.A.; Fletcher, B.; Watson, P.; Jones, F.A.; Miller, L.; Scott, G.M. Discrepancy between self-reported and observed hand hygiene behaviour in healthcare professionals. J. Hosp. Infect. 2006, 63, 418–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, C.; Tully, M.; Cooke, J. An investigation into the content validity of the Antimicrobial Self-Assessment Toolkit for NHS Trusts (ASAT v15a) using cognitive interviews with antimicrobial pharmacists. J. Clin. Pharm. Ther. 2015, 40, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Ryves, R.; Eyles, C.; Moore, M.; McDermott, L.; Little, P.; Leydon, G.M. Understanding the delayed prescribing of antibiotics for respiratory tract infection in primary care: A qualitative analysis. BMJ Open 2016, 6, e011882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, A.J.; Chen, M.-K.; Goldblatt, E.; Klatt, M.; Kligler, B.; Koithan, M.S.; Kreitzer, M.J.; Lee, J.K.; Lopez, A.M.; Maizes, V.; et al. Introducing integrative primary health care to an interprofessional audience: Feasibility and impact of an asynchronous online course. EXPLORE 2019. [Google Scholar] [CrossRef] [PubMed]

| Number | Question | November 2014–June 2016 | July 2016–September 2019 | Percentage Change |
|---|---|---|---|---|
| 1 | Do you use national or local antibiotic guidance when considering how to treat common infections? | 98% | 98% | No change |
| 2 | Do you analyse and discuss antibiotic prescribing at your practice in comparison to local indicators at least once a year? | 67% | 73% | 6% increase |
| 3 | Do you use patient-focused strategies to highlight the importance of responsible antibiotic use? For example, videos and posters in clinical and waiting areas. | 71% | 94% | 23% increase |
| 5 | Have you been involved in a practice antibiotic audit in the last two years? | 45% | 98% | 53% increase |
| 6 | Do you usually record clinical indications for prescribed antibiotics in patient notes using Read codes or Snomed codes? | 75% | 80% | 5% increase |
| 8 | Is the latest antibiotic guidance made available to all temporary prescribers working in your surgery? | 63% | 55% | 8% decrease |
| 9 | Do you use back-up/delayed prescribing? | 94% | 99% | 5% increase |
| 12 | Do you keep a written record and surgery action plan resulting from antibiotic audits? | 62% | 81% | 19% increase |
| 13 | Do you have a strategy to avoid patients reconsulting with other clinicians to obtain antibiotics? | 33% | 45% | 12% increase |
| 14 | Have you undertaken any antibiotic-related prescribing clinician courses, for example the TARGET webinars, ‘managing acute respiratory tract infections’ and ‘managing UTIs elearning courses on the RCGP online learning environment within the past two years? | 29% | 37% | 8% increase |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, L.F.; Verlander, N.Q.; Lecky, D.M.; Altaf, S.; Pilat, D.; McNulty, C. Self-Reported Antimicrobial Stewardship Practices in Primary Care Using the TARGET Antibiotics Self-Assessment Tool. Antibiotics 2020, 9, 253. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050253
Jones LF, Verlander NQ, Lecky DM, Altaf S, Pilat D, McNulty C. Self-Reported Antimicrobial Stewardship Practices in Primary Care Using the TARGET Antibiotics Self-Assessment Tool. Antibiotics. 2020; 9(5):253. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050253
Chicago/Turabian StyleJones, Leah Ffion, Neville Quinton Verlander, Donna Marie Lecky, Sabeen Altaf, Dirk Pilat, and Cliodna McNulty. 2020. "Self-Reported Antimicrobial Stewardship Practices in Primary Care Using the TARGET Antibiotics Self-Assessment Tool" Antibiotics 9, no. 5: 253. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050253







