Multicenter, Observational Cohort Study Evaluating Third-Generation Cephalosporin Therapy for Bloodstream Infections Secondary to Enterobacter, Serratia, and Citrobacter Species
Abstract
:1. Introduction
2. Results
2.1. Participants
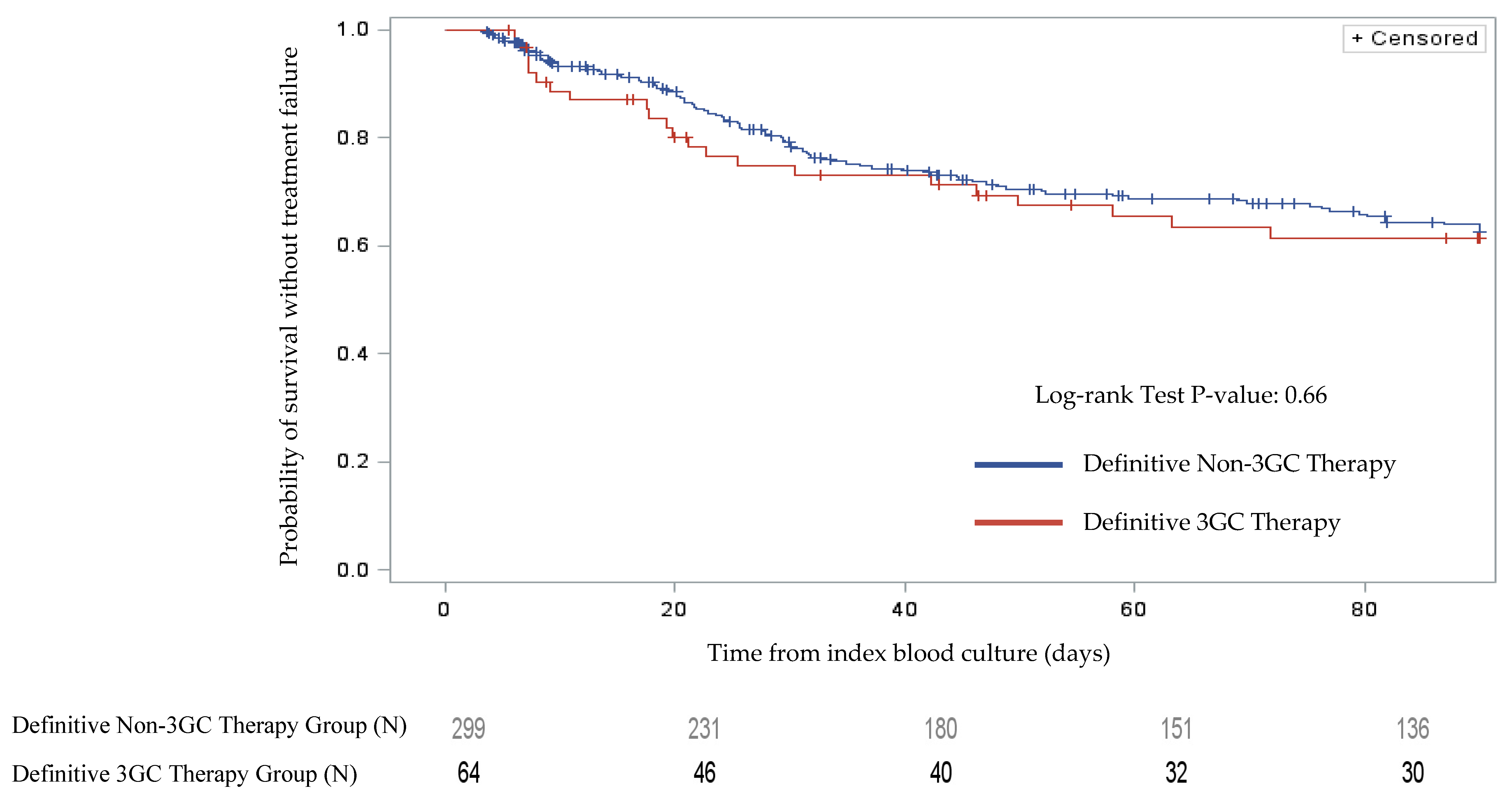
2.2. Clinical Outcomes
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Study Definitions
4.3. Study Endpoints
4.4. Microbiology Procedures & Data Management
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-treat resistance in Gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacoby, G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.N.; Ferguson, J.K. Antibiotic therapy for inducible AmpC beta-lactamase-producing Gram-negative bacilli: What are the alternatives to carbapenems, quinolones and aminoglycosides? Int. J. Antimicrob. Agents 2012, 40, 297–305. [Google Scholar] [CrossRef]
- Dunne, W.M., Jr.; Hardin, D.J. Use of several inducer and substrate antibiotic combinations in a disk approximation assay format to screen for AmpC induction in patient isolates of Pseudomonas aeruginosa, Enterobacter spp., Citrobacter spp., and Serratia spp. J. Clin. Microbiol. 2005, 43, 5945–5949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, J.W.; Fine, M.J.; Shlaes, D.M.; Quinn, J.P.; Hooper, D.C.; Johnson, M.P.; Ramphal, R.; Wagener, M.M.; Miyashiro, D.K.; Yu, V.L. Enterobacter bacteremia: Clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 1991, 115, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Cosgrove, S.; Harris, A.; Eliopoulos, G.M.; Carmeli, Y. Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob. Agents Chemother. 2001, 45, 2628–2630. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.I.; Kim, S.H.; Park, W.B.; Lee, K.D.; Kim, H.B.; Oh, M.D.; Kim, E.C.; Choe, K.W. Bloodstream infections caused by Enterobacter species: Predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin. Infect. Dis. 2004, 39, 812–818. [Google Scholar] [CrossRef]
- Yang, K.; Guglielmo, B.J. Diagnosis and treatment of extended-spectrum and AmpC beta-lactamase-producing organisms. Ann. Pharmacother. 2007, 41, 1427–1435. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.E.; Park, S.J.; Choi, S.H.; Lee, S.O.; Jeong, J.Y.; Kim, M.N.; Woo, J.H.; Kim, Y.S. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: Implications for antibiotic use. Antimicrob. Agents Chemother. 2008, 52, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.N.; Wei, J.Y.; Shen, A.W.; Abdile, A.A.; Paynter, S.; Huxley, R.R.; Pandeya, N.; Doi, Y.; Huh, K.; O’Neal, C.S.; et al. Carbapenems versus alternative antibiotics for the treatment of bloodstream infections caused by Enterobacter, Citrobacter or Serratia species: A systematic review with meta-analysis. J. Antimicrob. Chemother. 2016, 71, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Mizrahi, A.; Delerue, T.; Morel, H.; Le Monnier, A.; Carbonnelle, E.; Pilmis, B.; Zahar, J.R.; on behalf the Saint-Joseph/Avicenna Study Group. Infections caused by naturally AmpC-producing Enterobacteriaceae: Can we use third-generation cephalosporins? A narrative review. Int. J. Antimicrob. Agents 2020, 55, 105834. [Google Scholar] [CrossRef] [PubMed]
- Meini, S.; Tascini, C.; Cei, M.; Sozio, E.; Rossolini, G.M. AmpC β-lactamase-producing Enterobacterales: What a clinician should know. Infection 2019, 47, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Sendi, P.; Seiffert, S.N.; Droz, S.; Perreten, V.; Hujer, A.M.; Bonomo, R.A.; Mühlemann, K.; Endimiani, A. Characterisation and clinical features of Enterobacter cloacae bloodstream infections occurring at a tertiary care university hospital in Switzerland: Is cefepime adequate therapy? Int. J. Antimicrob. Agents 2013, 41, 236–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siedner, M.J.; Galar, A.; Guzman-Suarez, B.B.; Kubiak, D.W.; Baghdady, N.; Ferraro, M.J.; Hooper, D.C.; O’Brien, T.F.; Marty, F.M. Cefepime vs. other antibacterial agents for the treatment of Enterobacter species bacteremia. Clin. Infect. Dis. 2014, 58, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Tamma, P.D.; Girdwood, S.C.; Gopaul, R.; Tekle, T.; Roberts, A.A.; Harris, A.D.; Cosgrove, S.E.; Carroll, K.C. The use of cefepime for treating AmpC beta-lactamase-producing Enterobacteriaceae. Clin. Infect. Dis. 2013, 57, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.N.A.; Peri, A.M.; Pelecanos, A.M.; Hughes, C.M.; Paterson, D.L.; Ferguson, J.K. Risk factors for relapse or persistence of bacteraemia caused by Enterobacter spp.: A case-control study. Antimicrob. Resist. Infect. Control 2017, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- MacVane, S.H.; Nolte, F.S. Benefits of adding a rapid PCR-based blood culture identification panel to an established antimicrobial stewardship program. J. Clin. Microbiol. 2016, 54, 2455–2463. [Google Scholar] [CrossRef] [Green Version]
- Kutob, L.F.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Albrecht, H.; Al-Hasan, M.N. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int. J. Antimicrob. Agents 2016, 48, 498–503. [Google Scholar] [CrossRef]
- Punjabi, C.; Tien, V.; Meng, L.; Deresinski, S.; Holubar, M. Oral fluoroquinolone or trimethoprim-sulfamethoxazole vs. ß-lactams as step-down therapy for Enterobacteriaceae bacteremia: Systematic review and meta-analysis. In Open Forum Infectious Diseases; Oxford University Press: Oxford, MS, USA, 2019. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, C.C.; Li, C.W.; Li, M.C.; Chen, P.L.; Chang, C.M.; Ko, W.C. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: Unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob. Agents Chemother. 2015, 59, 7558–7563. [Google Scholar] [CrossRef] [Green Version]
- Al-Hasan, M.N.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Predictive scoring model of mortality in Gram-negative bloodstream infection. Clin. Microbiol. Infect. 2013, 19, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Al-Hasan, M.N.; Juhn, Y.J.; Bang, D.W.; Yang, H.J.; Baddour, L.M. External validation of bloodstream infection mortality risk score in a population-based cohort. Clin. Microbiol. Infect. 2014, 20, 886–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hasan, M.N.; Lahr, B.D.; Eckel-Passow, J.E.; Baddour, L.M. Temporal trends in Enterobacter species bloodstream infection: A population-based study from 1998–2007. Clin. Microbiol. Infect. 2011, 17, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, K.L.; Stoessel, A.; Justo, J.A.; Bookstaver, P.B.; Kohn, J.; Derrick, C.B.; Albrecht, H.; Al-Hasan, M.N. Association between chronic hemodialysis and bloodstream infections caused by chromosomally mediated AmpC-producing Enterobacteriaceae. Am. J. Infect. Control 2016, 44, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Baron, E.J.; Miller, J.M.; Weinstein, M.P.; Richter, S.S.; Gilligan, P.H.; Thomson, R.B., Jr.; Bourbeau, P.; Carroll, K.C.; Kehl, S.C.; Dunne, W.M.; et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2013 recommendations by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM)(a). Clin. Infect. Dis. 2013, 57, e22–e121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cain, S.E.; Kohn, J.; Bookstaver, P.B.; Albrecht, H.; Al-Hasan, M.N. Stratification of the impact of inappropriate empirical antimicrobial therapy for Gram-negative bloodstream infections by predicted prognosis. Antimicrob. Agents Chemother. 2015, 59, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
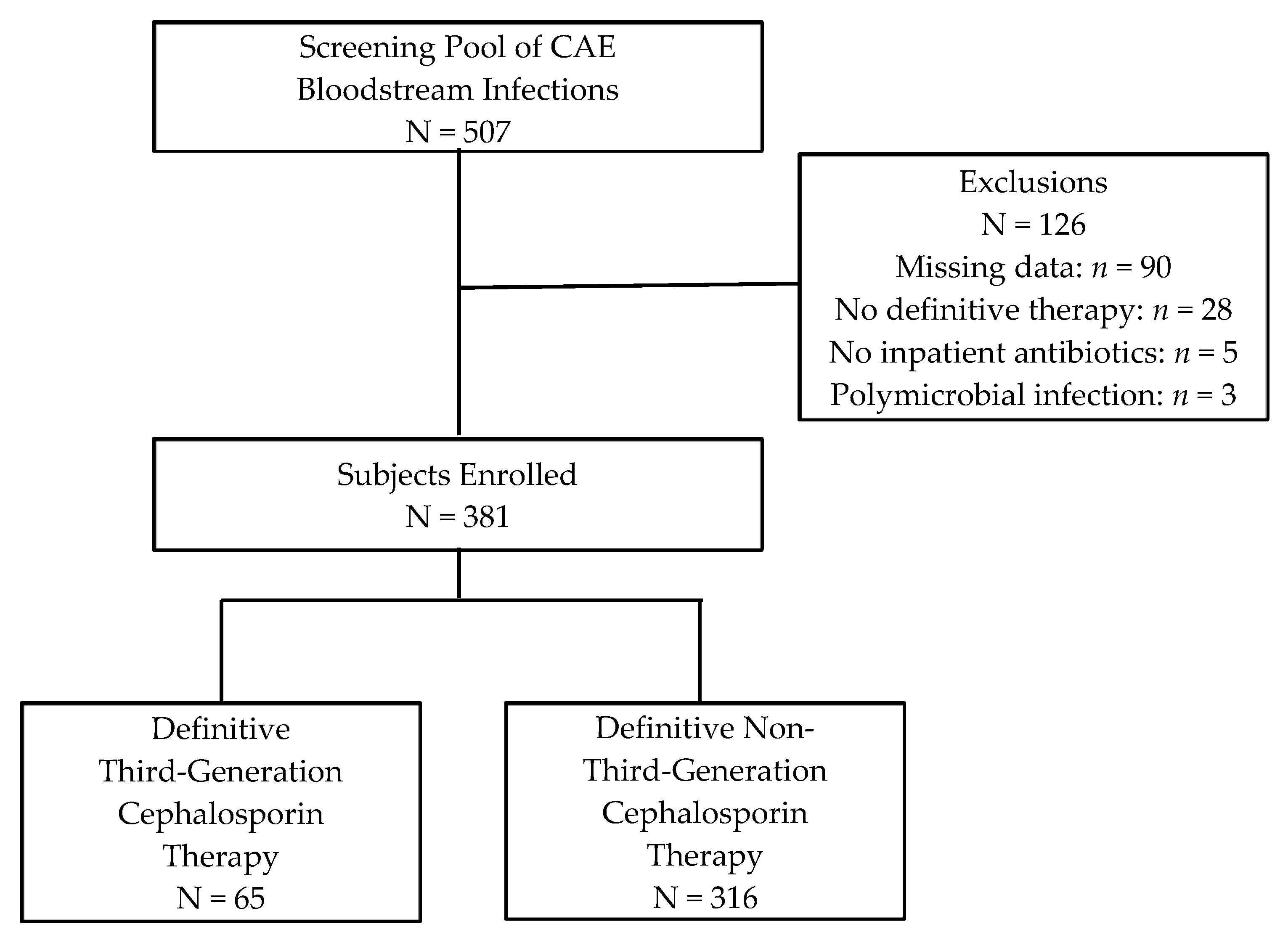
| Characteristic | Definitive 3GC Therapy a N = 65 | Definitive Non-3GC Therapy a N = 316 | p-Value |
|---|---|---|---|
| Age, y (median (IQR)) | 59 (46.5–68.0) | 60 (47.8–70.0) | 0.989 |
| Male | 32 (49.2) | 202 (63.9) | 0.027 |
| Weight, kg (median (IQR)) | 80 (71.0–102.3) | 85 (70.0–103.0) | 0.884 |
| Race/Ethnicity | 0.308 | ||
| Black | 33 (50.8) | 130 (41.4) | |
| White | 29 (44.6) | 175 (55.4) | |
| Other/Unknown | 3 (4.6) | 11 (3.5) | |
| Intensive Care Unit Admission | 19 (29.2) | 144 (45.6) | 0.016 |
| CrCl, mL/min (median (IQR)) | 45 (21.9–85.4) | 56 (28.7–86.7) | 0.036 |
| Penicillin/Cephalosporin Allergy | 8 (12.3) | 41 (13.0) | 0.884 |
| Charlson Comorbidity Index (median (IQR)) | 3 (2.0–6.0) | 4 (2.0–6.0) | 0.984 |
| Source of Infection | |||
| Central Venous Catheter | 17 (26.2) | 61 (19.3) | 0.213 |
| Urinary Tract | 15 (23.1) | 63 (19.9) | 0.568 |
| Skin- or Skin Structure-Related | 9 (13.9) | 35 (11.1) | 0.525 |
| Intra-Abdominal | 6 (9.2) | 34 (10.8) | 0.714 |
| Respiratory | 3 (4.6) | 42 (13.3) | 0.049 |
| Other | 6 (9.2) | 16 (5.1) | 0.237 |
| Unknown Source | 9 (13.9) | 76 (24.1) | 0.072 |
| Site of Acquisition | 0.275 | ||
| Hospital-Acquired | 27 (42.2) | 160 (50.6) | |
| Healthcare-Associated | 21 (32.8) | 102 (32.3) | |
| Community-Acquired | 16 (25.0) | 54 (17.1) | |
| Pitt Bacteremia Score (median (IQR)) | 2 (1.0–3.0) | 3 (1.0–4.0) | 0.043 |
| Adequate Empiric Therapy | 60 (92.3) | 291 (92.1) | 0.952 |
| Organism | Total a N = 381 | Definitive 3GC Therapy a,b N = 65 | Definitive Non-3GC Therapy a,b N = 316 |
|---|---|---|---|
| Enterobacter spp. | 238 (62.5) | 33 (50.7) | 205 (64.8) |
| E. cloacae | 142 (37.3) | 19 (29.2) | 123 (38.9) |
| E. aerogenes | 82 (21.5) | 14 (21.5) | 68 (21.5) |
| Other Enterobacter spp. | 14 (3.7) | 0 (0) | 14 (4.4) |
| Serratia spp. | 104 (27.3) | 21 (32.3) | 83 (26.2) |
| S. marcescens | 100 (26.2) | 19 (29.2) | 81 (25.6) |
| Other Serratia spp. | 4 (1.0) | 2 (3.1) | 2 (0.6) |
| Citrobacter spp. | 39 (10.2) | 11 (16.9) | 28 (8.8) |
| C. freundii | 21 (5.5) | 8 (12.3) | 13 (4.1) |
| Other Citrobacter spp. | 18 (4.7) | 3 (4.6) | 15 (4.7) |
| Variable | Definitive 3GC Therapy a N = 65 | Definitive Non-3GC Therapy a N = 316 | p-Value |
|---|---|---|---|
| Number of Inpatient Antibiotics Used (mean (SD)) | 3.5 (1.6) | 3.0 (1.4) | 0.005 |
| Antibiotic Class | |||
| Extended-Spectrum Penicillins b | 30 (46.2) | 187 (59.2) | 0.054 |
| Third-Generation Cephalosporins | 65 (100) | 31 (9.8) c | <0.001 |
| Fourth-Generation Cephalosporins | 12 (18.5) | 83 (26.3) | 0.185 |
| Carbapenems | 16 (24.6) | 115 (36.4) | 0.069 |
| Fluoroquinolones | 25 (38.5) | 158 (50.0) | 0.090 |
| Aminoglycosides | 12 (18.5) | 54 (17.1) | 0.790 |
| Other Antibiotics | 40 (61.5) | 182 (57.6) | 0.580 |
| Inpatient Gram-Negative Antibiotic Duration, days (median (IQR)) | 10.3 (5.7–15.6) | 8.8 (5.0–13.9) | 0.578 |
| Empiric Duration | 2.8 (2.3–3.0) | 2.7 (2.2–3.0) | 0.470 |
| Definitive Duration | 7.4 (3.5–12.9) | 5.8 (2.4–11.0) | 0.620 |
| Inpatient 3GC Duration, days (median (IQR)) | 4.4 (2.8–7.4) | 0.5 (0.5–1.6) | <0.001 |
| Empiric 3GC Duration | 2.5 (0.9–3.0) | 0.5 (0.5–1.6) | 0.002 |
| Definitive 3GC Duration | 3.3 (1.4–6.5) | -- | -- |
| Proportion of Time 3GC Used for Definitive Inpatient Therapy (median (IQR)) | 0.7 (0.3–1.0) | -- | -- |
| Adequate Definitive Therapy d | 0.487 | ||
| Yes | 63 (98.4) | 281 (95.3) | |
| No | 1 (1.6) | 14 (4.7) | |
| Number of Patients Discharged on Antibiotics | 34 (52.3) | 161 (51.1) | 0.861 |
| Outcome | Definitive 3GC Therapy a N = 65 | Definitive Non-3GC Therapy a N = 316 | p-Value |
|---|---|---|---|
| Overall Treatment Failure | 22 (33.8) | 94 (29.7) | 0.513 |
| In-Hospital Mortality | 4 (6.2) | 33 (10.4) | 0.302 |
| Hospital Readmission within 30 Days | 15 (23.1) | 53 (17.0) | 0.245 |
| Reinfection within 90 Days | 6 (9.2) | 16 (5.1) | 0.232 |
| 30-Day Mortality | 4 (6.2) | 27 (8.5) | 0.521 |
| Length of Hospital Stay, days (median (IQR)) | 12.7 (7.7–32.6) | 14.2 (6.7–34.9) | 0.158 |
| Variable | Adjusted HR | 95% CI | p-Value |
|---|---|---|---|
| Definitive 3GC Therapy a | 0.93 | 0.51–1.72 | 0.825 |
| Adequate Empiric Therapy | 0.40 | 0.12–1.32 | 0.132 |
| Male Sex | 0.67 | 0.42–1.08 | 0.097 |
| CrCl, per mL/min increase | 1.00 | 0.99–1.08 | 0.223 |
| Source of Infection other than Urinary Tract or CVC | 1.04 | 0.65–1.67 | 0.858 |
| Site of Acquisition | |||
| Community-Acquired | Ref. | - | - |
| Healthcare-Associated | 0.85 | 0.46–1.57 | 0.596 |
| Hospital-Acquired | 1.13 | 0.59–2.17 | 0.714 |
| Malignancy | 1.71 | 0.98–2.99 | 0.059 |
| Liver Cirrhosis | 1.88 | 0.80–4.46 | 0.151 |
| Pitt Bacteremia Score, per point increase | 1.04 | 0.93–1.17 | 0.488 |
| Penicillin/Cephalosporin Allergy | 1.30 | 0.80–2.13 | 0.292 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derrick, C.; Bookstaver, P.B.; Lu, Z.K.; Bland, C.M.; King, S.T.; Stover, K.R.; Rumley, K.; MacVane, S.H.; Swindler, J.; Kincaid, S.; et al. Multicenter, Observational Cohort Study Evaluating Third-Generation Cephalosporin Therapy for Bloodstream Infections Secondary to Enterobacter, Serratia, and Citrobacter Species. Antibiotics 2020, 9, 254. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050254
Derrick C, Bookstaver PB, Lu ZK, Bland CM, King ST, Stover KR, Rumley K, MacVane SH, Swindler J, Kincaid S, et al. Multicenter, Observational Cohort Study Evaluating Third-Generation Cephalosporin Therapy for Bloodstream Infections Secondary to Enterobacter, Serratia, and Citrobacter Species. Antibiotics. 2020; 9(5):254. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050254
Chicago/Turabian StyleDerrick, Caroline, P. Brandon Bookstaver, Zhiqiang K. Lu, Christopher M. Bland, S. Travis King, Kayla R. Stover, Kathey Rumley, Shawn H. MacVane, Jenna Swindler, Scott Kincaid, and et al. 2020. "Multicenter, Observational Cohort Study Evaluating Third-Generation Cephalosporin Therapy for Bloodstream Infections Secondary to Enterobacter, Serratia, and Citrobacter Species" Antibiotics 9, no. 5: 254. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9050254






