The Antimicrobial Activity of Cannabinoids
Abstract
:1. Introduction
2. Antibacterial Effects of C. sativa Extracts
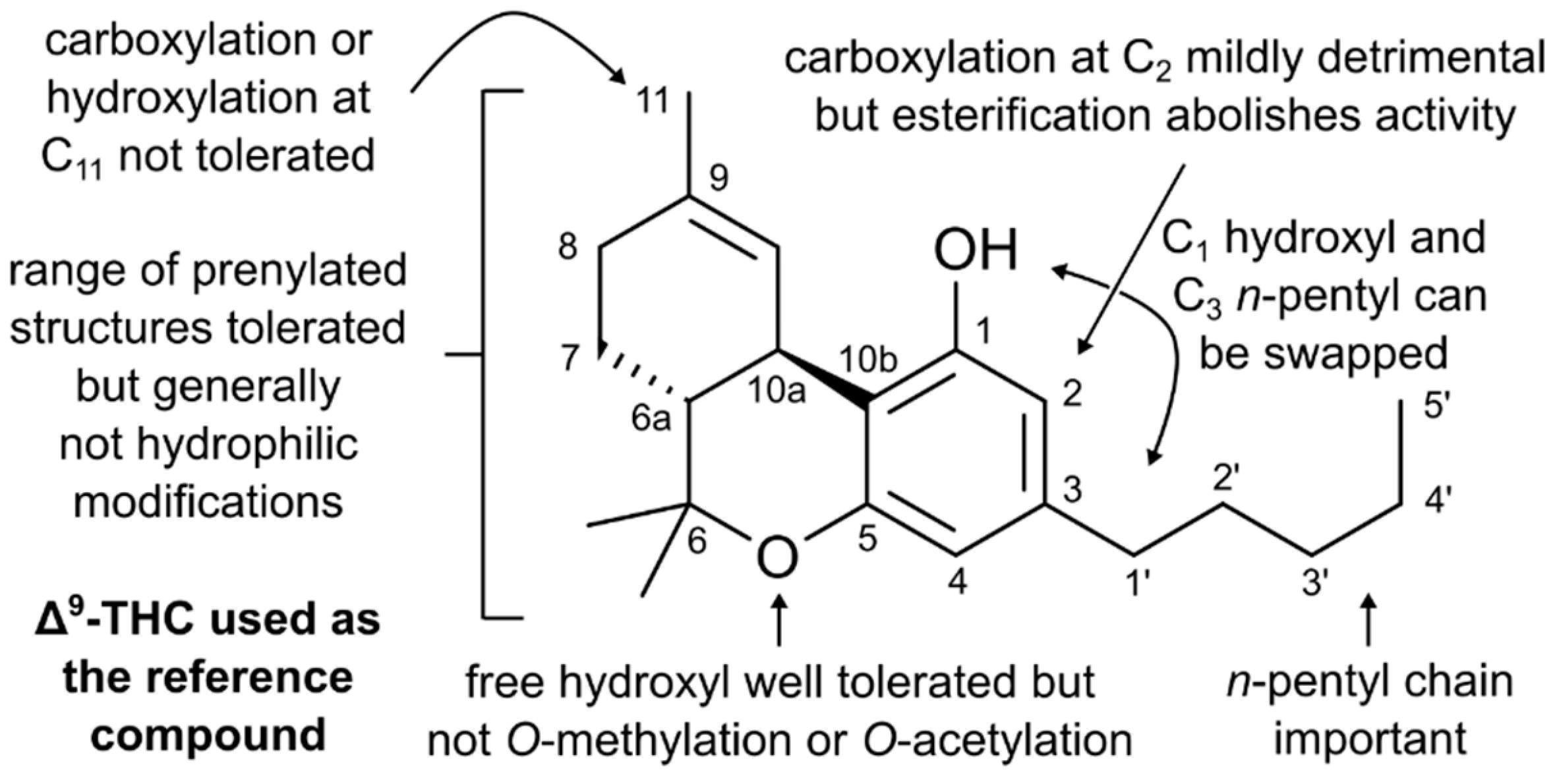
3. Structure–Activity Relationships of Cannabinoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance: London, UK, 2016; pp. 1–81. [Google Scholar]
- Gilbert, D.N.; Guidos, R.J.; Boucher, H.W.; Talbot, G.H.; Spellberg, B.; Edwards, J.E., Jr.; Scheld, W.M.; Bradley, J.S.; John, G.; Bartlett, J.G. The 10 x ’20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 50, 1081–1083. [Google Scholar] [CrossRef] [Green Version]
- Lodato, E.M.; Kaplan, W. Background Paper 6.1 Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Cooper, M.A.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Khan, T.A.; Ali, I.; Hassan, A.; Ali, H.; Ud, Z.; Din, Z.H.; Tabassum, S.; Saqib, A.M.; Rehman, M.U. In vitro antibacterial activity of Cannabis sativa leaf extracts to some selective pathogenicbacterial strains. Int. J. Biosci. 2014, 4, 65–70. [Google Scholar]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. World Drug Report 2004; United Nations Publications: New York, NY, USA, 2004; Volume 1. [Google Scholar]
- Klahn, P. Cannabinoids-Promising antimicrobial drugs or intoxicants with benefits? Antibiotics 2020, 9, 297. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products/Progrès dans la Chimie des Substances Organiques Naturelles; Springer: Wien, Austria, 1967; pp. 175–213. [Google Scholar]
- Fischedick, J.T.; Hazekamp, A.; Erkelens, T.; Choi, Y.H.; Verpoorte, R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry 2010, 71, 2058–2073. [Google Scholar] [CrossRef]
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International union of pharmacology. XXVII. classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Głodowska, M.; Łyszcz, M. Cannabis sativa L. and its antimicrobial properties—A review. In Badania i Rozwój Młodych Naukowców w Polsce–Agronomia i Ochrona Roślin; Leśny, J., Chojnicki, B., Panfil, M., Nyćkowiak, J., Eds.; Młodzi Naukowcy: Poznań, Poland, 2017; pp. 77–82. [Google Scholar]
- Hernández-Cervantes, R.; Méndez-Díaz, M.; Prospéro-García, Ó.; Morales-Montor, J. Immunoregulatory role of cannabinoids during infectious disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Ozturk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.M.; Fowler, C.J. The endocannabinoid system: Drug targets, lead compounds, and potential therapeutic applications. J. Med. Chem. 2005, 48, 5059–5087. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, M.A.P.; Hindocha, C.; Green, S.F.; Wall, M.B.; Lees, R.; Petrilli, K.; Costello, H.; Ogunbiyi, M.O.; Bossong, M.G.; Freeman, T.P. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019, 195, 132–161. [Google Scholar] [CrossRef]
- Stout, S.M.; Cimino, N.M. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: A systematic review. Drug Metab. Rev. 2014, 46, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.; Pryce, G.; Giovannoni, G.; Thompson, A.J. The therapeutic potential of cannabis. Lancet Neurol. 2003, 2, 291–298. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The National Academies Collection: Reports funded by National Institutes of Health. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar] [CrossRef] [Green Version]
- Vogel, L. Cautious first guidance for prescribing pot. Can. Med. Assoc. J. 2014, 186, E595. [Google Scholar] [CrossRef] [Green Version]
- Lake, S.; Kerr, T.; Montaner, J. Prescribing medical cannabis in Canada: Are we being too cautious? Can. J. Public Health 2015, 106, 328–330. [Google Scholar] [CrossRef] [Green Version]
- Murray, R.M.; Morrison, P.D.; Henquet, C.; Forti, M.D. Cannabis, the mind and society: The hash realities. Nat. Rev. Neurosci. 2007, 8, 885. [Google Scholar] [CrossRef]
- Hall, W.; Solowij, N.; Lemon, J. The Health and Psychological Effects of Cannabis (National Drug Strategy Monograph No. 25); Australian Government Publishing Service: Canberra, Australia, 1994. [Google Scholar]
- Broyd, S.J.; van Hell, H.H.; Beale, C.; Yuecel, M.; Solowij, N. Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biol. Psychiatry 2016, 79, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Curran, H.V.; Brignell, C.; Fletcher, S.; Middleton, P.; Henry, J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002, 164, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Crean, R.D.; Tapert, S.F.; Minassian, A.; MacDonald, K.; Crane, N.A.; Mason, B.J. Effects of chronic, heavy cannabis use on executive functions. J. Addict. Med. 2011, 5, 9. [Google Scholar] [CrossRef]
- Dorard, G.; Berthoz, S.; Phan, O.; Corcos, M.; Bungener, C. Affect dysregulation in cannabis abusers. Eur. Child Adolesc. Psychiatry 2008, 17, 274–282. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Health and Social Effects of Nonmedical Cannabis Use; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Pacher, P.; Steffens, S.; Haskó, G.; Schindler, T.H.; Kunos, G. Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly. Nat. Rev. Cardiol. 2018, 15, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Perucca, E. Cannabinoids in the treatment of epilepsy: Hard evidence at last? J. Epilepsy Res. 2017, 7, 61–76. [Google Scholar] [CrossRef]
- Stockings, E.; Zagic, D.; Campbell, G.; Weier, M.; Hall, W.D.; Nielsen, S.; Herkes, G.K.; Farrell, M.; Degenhardt, L. Evidence for cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry 2018, 89, 741–753. [Google Scholar] [CrossRef]
- Suraev, A.S.; Todd, L.; Bowen, M.T.; Allsop, D.J.; McGregor, I.S.; Ireland, C.; Lintzeris, N. An Australian nationwide survey on medicinal cannabis use for epilepsy: History of antiepileptic drug treatment predicts medicinal cannabis use. Epilepsy Behav. 2017, 70, 334–340. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Campbell, F.A.; Tramèr, M.R.; Carroll, D.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 2001, 323, 13. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.A.; Azariah, F.; Lavender, V.T.; Stoner, N.S.; Bettiol, S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst. Rev. 2015, 11, CD009464. [Google Scholar] [CrossRef] [Green Version]
- Tramèr, M.R.; Carroll, D.; Campbell, F.A.; Reynolds, D.J.M.; Moore, R.A.; McQuay, H.J. Cannabinoids for control of chemotherapy induced nausea and vomiting: Quantitative systematic review. BMJ 2001, 323, 16. [Google Scholar]
- Kirkham, T.C. Cannabinoids and appetite: Food craving and food pleasure. Int. Rev. Psychiatry 2009, 21, 163–171. [Google Scholar] [CrossRef]
- Krejci, Z. Hemp (Cannabis sativa)—Antibiotic drugs. II. Method & results of bacteriological experiments & preliminary clinical experience. Die Pharm. 1958, 13, 155–166. [Google Scholar]
- Rabinovich, A.S.; BIu, A.; Zelepukha, S.I. Isolation and investigation of antibacterial properties of preparations from wild hemp (Cannabis ruderalis) growing in the Ukraine. Mikrobiolohichnyi Zhurnal 1959, 21, 40–48. [Google Scholar]
- Van Klingeren, B.; Ten Ham, M. Antibacterial activity of Δ 9-tetrahydrocannabinol and cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Singh, A.; Kitts, D.D.; Singh, A.P. Hemp (Cannabis Sativa L.) extract: Anti-Microbial properties, methods of extraction, and potential oral delivery. Food Rev. Int. 2019, 35, 664–684. [Google Scholar] [CrossRef]
- Novak, J.; Zitterl-Eglseer, K.; Deans, S.G.; Franz, C.M. Essential oils of different cultivars of Cannabis sativa L. And their antimicrobial activity. Flavour Fragr. J. 2001, 16, 259–262. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Almagboul, A.Z.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Lone, T.A.; Lone, R.A. Extraction of cannabinoids from Cannabis sativa L. plant and its potential antimicrobial activity. Univers. J. Med. Dent. 2012, 1, 51–55. [Google Scholar]
- Sarmadyan, H.; Solhi, H.; Hajimir, T.; Najarian-Araghi, N.; Ghaznavi-Rad, E. Determination of the antimicrobial effects of hydro-alcoholic extract of cannabis sativa on multiple drug resistant bacteria isolated from nosocomial infections. Iran. J. Toxicol. 2014, 7, 967–972. [Google Scholar]
- Vu, T.T.; Kim, H.; Tran, V.K.; Le Dang, Q.; Nguyen, H.T.; Kim, H.; Kim, I.S.; Choi, G.J.; Kim, J.C. In vitro antibacterial activity of selected medicinal plants traditionally used in Vietnam against human pathogenic bacteria. BMC Complement. Altern. Med. 2016, 16, 32. [Google Scholar] [CrossRef] [Green Version]
- Lelario, F.; Scrano, L.; Franchi, S.D.; Bonomo, M.G.; Salzano, G.; Milan, S.; Milella, L.; Bufo, S.A. Identification and antimicrobial activity of most representative secondary metabolites from different plant species. Chem. Biol. Technol. Agric. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and properties of hemp seed oil and its emulsions. Molecules 2017, 22, 700. [Google Scholar]
- Chakraborty, S.; Afaq, N.; Singh, N.; Majumdar, S. Antimicrobial activity of Cannabis sativa, Thuja orientalis and Psidium guajava leaf extracts against methicillin-resistant Staphylococcus aureus. J. Integr. Med. 2018, 16, 350–357. [Google Scholar] [CrossRef]
- Frassinetti, S.; Gabriele, M.; Moccia, E.; Longo, V.; Gioia, D.D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. LWT—Food Sci. Technol. 2020, 124, 109149. [Google Scholar] [CrossRef]
- Stahl, V.; Vasudevan, K. Comparison of efficacy of cannabinoids versus commercial oral care products in reducing bacterial content from dental plaque: A preliminary observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Lee, M.Y. The ameliorative effect of hemp seed hexane extracts on the propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef] [Green Version]
- Nadir, I.; Rana, N.F.; Ahmad, N.M.; Tanweer, T.; Batool, A.; Taimoor, Z.; Riaz, S.; Ali, S.M. Cannabinoids and terpenes as an antibacterial and antibiofouling promotor for pes water filtration membranes. Molecules 2020, 25, 691. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.E.; Elsohly, M.A. Biological activity of cannabichromene, its homologs and isomers. J. Clin. Pharmacol. 1981, 21, 283s–291s. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, purification, and antimicrobial characterization of cannabidiolic acid and cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Antimicrobial potential of endocannabinoid and endocannabinoid-like compounds against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2018, 8, 17696. [Google Scholar] [CrossRef]
- Feldman, M.; Smoum, R.; Mechoulam, R.; Steinberg, D. Potential combinations of endocannabinoid/endocannabinoid-like compounds and antibiotics against methicillin-resistant Staphylococcus aureus. PLoS ONE 2020, 15, e0231583. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, C.S.; Hojrup, P.; Klitgaard, J.K. Cannabidiol is an effective helper compound in combination with bacitracin to kill Gram-positive bacteria. Sci. Rep. 2020, 10, 4112. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol Is a novel modulator of bacterial membrane vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9070406
Karas JA, Wong LJM, Paulin OKA, Mazeh AC, Hussein MH, Li J, Velkov T. The Antimicrobial Activity of Cannabinoids. Antibiotics. 2020; 9(7):406. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9070406
Chicago/Turabian StyleKaras, John A., Labell J. M. Wong, Olivia K. A. Paulin, Amna C. Mazeh, Maytham H. Hussein, Jian Li, and Tony Velkov. 2020. "The Antimicrobial Activity of Cannabinoids" Antibiotics 9, no. 7: 406. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9070406




