Selective Eradication of Staphylococcus aureus by the Designer Genetically Programmed Yeast Biocontrol Agent
Abstract
:1. Introduction
2. Results
2.1. Constitutive Production of Recombinant Lysostaphin in P. pastoris
2.2. The Anti-Stapylococcal Activity of Engineered rLys P. pastoris
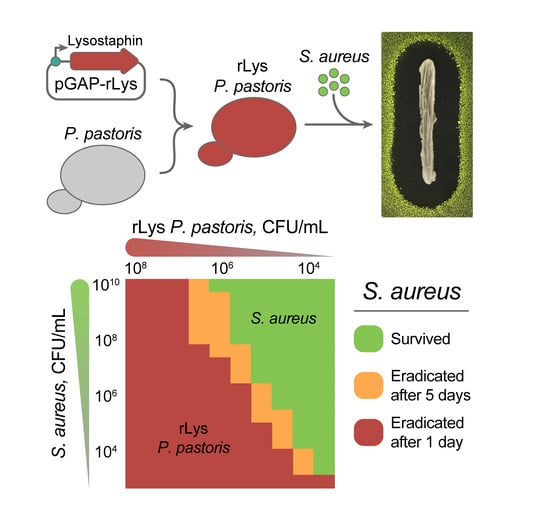
2.3. Eradication of S. aureus by Live rLys P. pastoris Biocontrol Agent
3. Discussion
4. Materials and Methods
4.1. Bacterial and Yeast Strains
4.2. Cultivation Conditions
4.3. Plasmid Construction and Yeast Transformation
4.4. Recombinant Lysostaphin Production
4.5. Agar Overlay Assay
4.6. Estimation of Lysostaphin Activity in Culture Media
4.7. Cocultivation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.R.; Feil, E.J.; Holden, M.T.G.; Quail, M.A.; Nickerson, E.K.; Chantratita, N.; Gardete, S.; Tavares, A.; Day, N.; Lindsay, J.A.; et al. Evolution of MRSA During Hospital Transmission and Intercontinental Spread. Science 2010, 327, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochman, H.; Elwyn, S.; Moran, N.A. Calibrating bacterial evolution. Proc. Natl. Acad. Sci. USA 1999, 96, 12638–12643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, B.; Wilson, D.J.; Feil, E.; Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proc. Biol. Sci. 2018, 285, 20180789. [Google Scholar] [CrossRef]
- Waters, E.M.; Rowe, S.E.; O’Gara, J.P.; Conlon, B.P. Convergence of Staphylococcus aureus Persister and Biofilm Research: Can Biofilms Be Defined as Communities of Adherent Persister Cells? PLoS Pathog. 2016, 12, e1006012. [Google Scholar] [CrossRef]
- Wu, J.A.; Kusuma, C.; Mond, J.J.; Kokai-Kun, J.F. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 2003, 47, 3407–3414. [Google Scholar] [CrossRef] [Green Version]
- Schindler, C.A.; Schuhardt, V.T. Lysostaphin: A New Bacteriolytic Agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA 1964, 51, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Delgado, L.S.; Walters-Morgan, H.; Salamaga, B.; Robertson, A.J.; Hounslow, A.M.; Jagielska, E.; Sabała, I.; Williamson, M.P.; Lovering, A.L.; Mesnage, S. Two-site recognition of Staphylococcus aureus peptidoglycan by lysostaphin SH3b. Nat. Chem. Biol. 2020, 16, 24–30. [Google Scholar] [CrossRef]
- Bastos, M.D.; Coutinho, B.G.; Coelho, M.L. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals 2010, 3, 1139–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitman, W.B. Staphylococcus. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–43. [Google Scholar] [CrossRef]
- Kusuma, C.M.; Kokai-Kun, J.F. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3256–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.Y.; Li, C.R.; Lou, R.H.; Wang, Y.M.; Zhang, W.X.; Chen, H.Z.; Huang, Q.S.; Han, Y.X.; Jiang, J.D.; You, X.F. In vitro activity of recombinant lysostaphin against Staphylococcus aureus isolates from hospitals in Beijing, China. J. Med. Microbiol. 2007, 56, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Placencia, F.X.; Kong, L.; Weisman, L.E. Treatment of methicillin-resistant Staphylococcus aureus in neonatal mice: Lysostaphin versus vancomycin. Pediatr. Res. 2009, 65, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Dajcs, J.J.; Hume, E.B.; Moreau, J.M.; Caballero, A.R.; Cannon, B.M.; O’Callaghan, R.J. Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1432–1437. [Google Scholar]
- Climo, M.W.; Patron, R.L.; Goldstein, B.P.; Archer, G.L. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 1998, 42, 1355–1360. [Google Scholar] [CrossRef] [Green Version]
- Kokai-Kun, J.F.; Chanturiya, T.; Mond, J.J. Lysostaphin as a treatment for systemic Staphylococcus aureus infection in a mouse model. J. Antimicrob. Chemother. 2007, 60, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [Green Version]
- Kerr, D.E.; Plaut, K.; Bramley, A.J.; Williamson, C.M.; Lax, A.J.; Moore, K.; Wells, K.D.; Wall, R.J. Lysostaphin expression in mammary glands confers protection against staphylococcal infection in transgenic mice. Nat. Biotechnol. 2001, 19, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, P.; Woischnig, A.K.; Charpin-El Hamri, G.; Ye, H.; Folcher, M.; Xie, M.; Khanna, N.; Fussenegger, M. Immunomimetic Designer Cells Protect Mice from MRSA Infection. Cell 2018, 174, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazanovic, K.; Zhao, H.; Choi, Y.; Li, W.; Salvat, R.S.; Osipovitch, D.C.; Fields, J.; Moise, L.; Berwin, B.L.; Fiering, S.N.; et al. Structure-based redesign of lysostaphin yields potent antistaphylococcal enzymes that evade immune cell surveillance. Mol. Ther. Methods Clin. Dev. 2015, 2, 15021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Blazanovic, K.; Choi, Y.; Bailey-Kellogg, C.; Griswold, K.E. Gene and protein sequence optimization for high-level production of fully active and aglycosylated lysostaphin in Pichia pastoris. Appl. Environ. Microbiol. 2014, 80, 2746–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef] [Green Version]
- Terekhov, S.S.; Smirnov, I.V.; Malakhova, M.V.; Samoilov, A.E.; Manolov, A.I.; Nazarov, A.S.; Danilov, D.V.; Dubiley, S.A.; Osterman, I.A.; Rubtsova, M.P.; et al. Ultrahigh-throughput functional profiling of microbiota communities. Proc. Natl. Acad. Sci. USA 2018, 115, 9551–9556. [Google Scholar] [CrossRef] [Green Version]
- Terekhov, S.S.; Nazarov, A.S.; Mokrushina, Y.A.; Baranova, M.N.; Potapova, N.A.; Malakhova, M.V.; Ilina, E.N.; Smirnov, I.V.; Gabibov, A.G. Deep Functional Profiling Facilitates the Evaluation of the Antibacterial Potential of the Antibiotic Amicoumacin. Antibiotics 2020, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Watson, S.P.; Clements, M.O.; Foster, S.J. Characterization of the starvation-survival response of Staphylococcus aureus. J. Bacteriol. 1998, 180, 1750–1758. [Google Scholar] [CrossRef] [Green Version]
- Parenteau, J.; Maignon, L.; Berthoumieux, M.; Catala, M.; Gagnon, V.; Abou Elela, S. Introns are mediators of cell response to starvation. Nature 2019, 565, 612–617. [Google Scholar] [CrossRef]
- Petti, A.A.; Crutchfield, C.A.; Rabinowitz, J.D.; Botstein, D. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc. Natl. Acad. Sci. USA 2011, 108, E1089–E1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sideri, T.; Rallis, C.; Bitton, D.A.; Lages, B.M.; Suo, F.; Rodríguez-López, M.; Du, L.-L.; Bähler, J. Parallel profiling of fission yeast deletion mutants for proliferation and for lifespan during long-term quiescence. G3 2014, 5, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebnegger, C.; Vos, T.; Graf, A.B.; Valli, M.; Pronk, J.T.; Daran-Lapujade, P.; Mattanovich, D. Pichia pastoris Exhibits High Viability and a Low Maintenance Energy Requirement at Near-Zero Specific Growth Rates. Appl. Environ. Microbiol. 2016, 82, 4570–4583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, K.L.; Roberts, C.; Disz, T.; Vonstein, V.; Hwang, K.; Overbeek, R.; Olson, P.D.; Projan, S.J.; Dunman, P.M. Characterization of the Staphylococcus aureus Heat Shock, Cold Shock, Stringent, and SOS Responses and Their Effects on Log-Phase mRNA Turnover. J. Bacteriol. 2006, 188, 6739–6756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Xiong, F.; Lin, Q.; d’Anjou, M.; Daugulis, A.J.; Yang, D.S.C.; Hew, C.L. Low-Temperature Increases the Yield of Biologically Active Herring Antifreeze Protein in Pichia pastoris. Protein Expr. Purif. 2001, 21, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Yang, L.; Guo, Y.; Fang, F.; Wang, D.; Li, R.; Jiang, M.; Kang, W.; Ma, J.; Sun, J.; et al. High-temperature cultivation of recombinant Pichia pastoris increases endoplasmic reticulum stress and decreases production of human interleukin-10. Microbial. Cell Factories 2014, 13, 163. [Google Scholar] [CrossRef] [Green Version]
- Terekhov, S.S.; Mokrushina, Y.A.; Nazarov, A.S.; Zlobin, A.; Zalevsky, A.; Bourenkov, G.; Golovin, A.; Belogurov, A.; Osterman, I.A.; Kulikova, A.A.; et al. A kinase bioscavenger provides antibiotic resistance by extremely tight substrate binding. Sci. Adv. 2020, 6, eaaz9861. [Google Scholar] [CrossRef]
- Strandén, A.M.; Ehlert, K.; Labischinski, H.; Berger-Bächi, B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 1997, 179, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Ehlert, K.; Schröder, W.; Labischinski, H. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 1997, 179, 7573–7576. [Google Scholar] [CrossRef] [Green Version]
- Kusuma, C.; Jadanova, A.; Chanturiya, T.; Kokai-Kun, J.F. Lysostaphin-resistant variants of Staphylococcus aureus demonstrate reduced fitness in vitro and in vivo. Antimicrob. Agents Chemother. 2007, 51, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Climo, M.W.; Ehlert, K.; Archer, G.L. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1431–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiri, N.; Archer, G.; Climo, M.W. Combinations of lysostaphin with beta-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2002, 46, 2017–2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Letchworth, G.J. High efficiency transformation by electroporation of Pichia pastoris pretreated with lithium acetate and dithiothreitol. BioTechniques 2004, 36, 152–154. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pipiya, S.O.; Mokrushina, Y.A.; Gabibov, A.G.; Smirnov, I.V.; Terekhov, S.S. Selective Eradication of Staphylococcus aureus by the Designer Genetically Programmed Yeast Biocontrol Agent. Antibiotics 2020, 9, 527. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090527
Pipiya SO, Mokrushina YA, Gabibov AG, Smirnov IV, Terekhov SS. Selective Eradication of Staphylococcus aureus by the Designer Genetically Programmed Yeast Biocontrol Agent. Antibiotics. 2020; 9(9):527. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090527
Chicago/Turabian StylePipiya, Sofiya O., Yuliana A. Mokrushina, Alexander G. Gabibov, Ivan V. Smirnov, and Stanislav S. Terekhov. 2020. "Selective Eradication of Staphylococcus aureus by the Designer Genetically Programmed Yeast Biocontrol Agent" Antibiotics 9, no. 9: 527. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090527