Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria
Abstract
:1. Introduction
2. Experimental Methodology
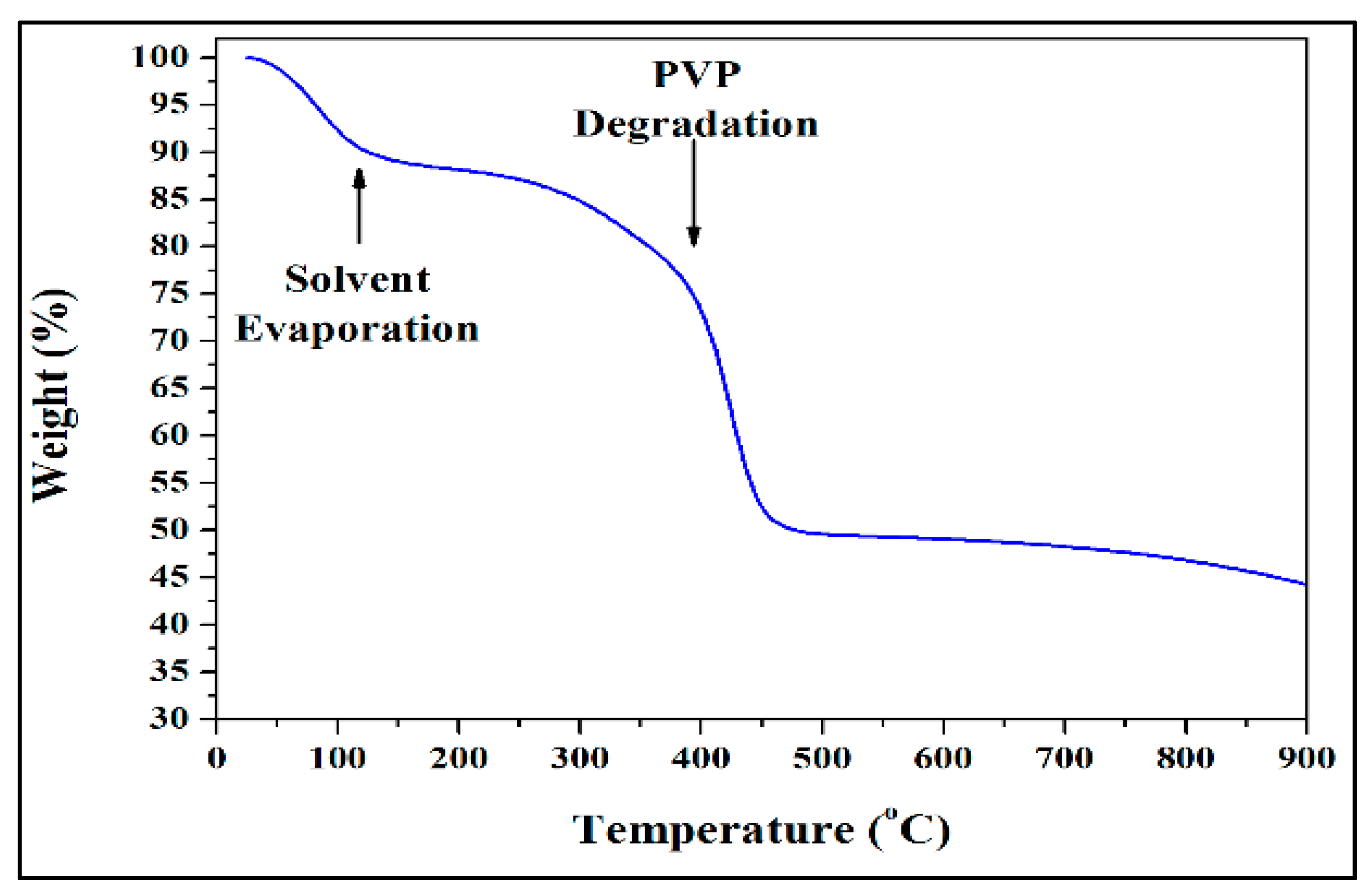
2.1. Electrospinning and Heating Protocol
2.2. Characterization of Electrospun TiO2 NFs
2.3. Evaluation of Antibacterial Activity of Electrospun TiO2 NFs
2.3.1. Bacterial Culture
2.3.2. Investigation of Minimum Inhibitory and Minimum Bactericidal Concentration (MIC/MBC) Values of Electrospun TiO2 NFs
2.4. Effect of TiO2 NFs on Biofilm Formation
2.5. Effect of TiO2 NFs on the Morphology of P. aeruginosa and MRSA: SEM Analysis
3. Results and Discussion
3.1. Effects of the Calcining Atmosphere on TiO2 Colour
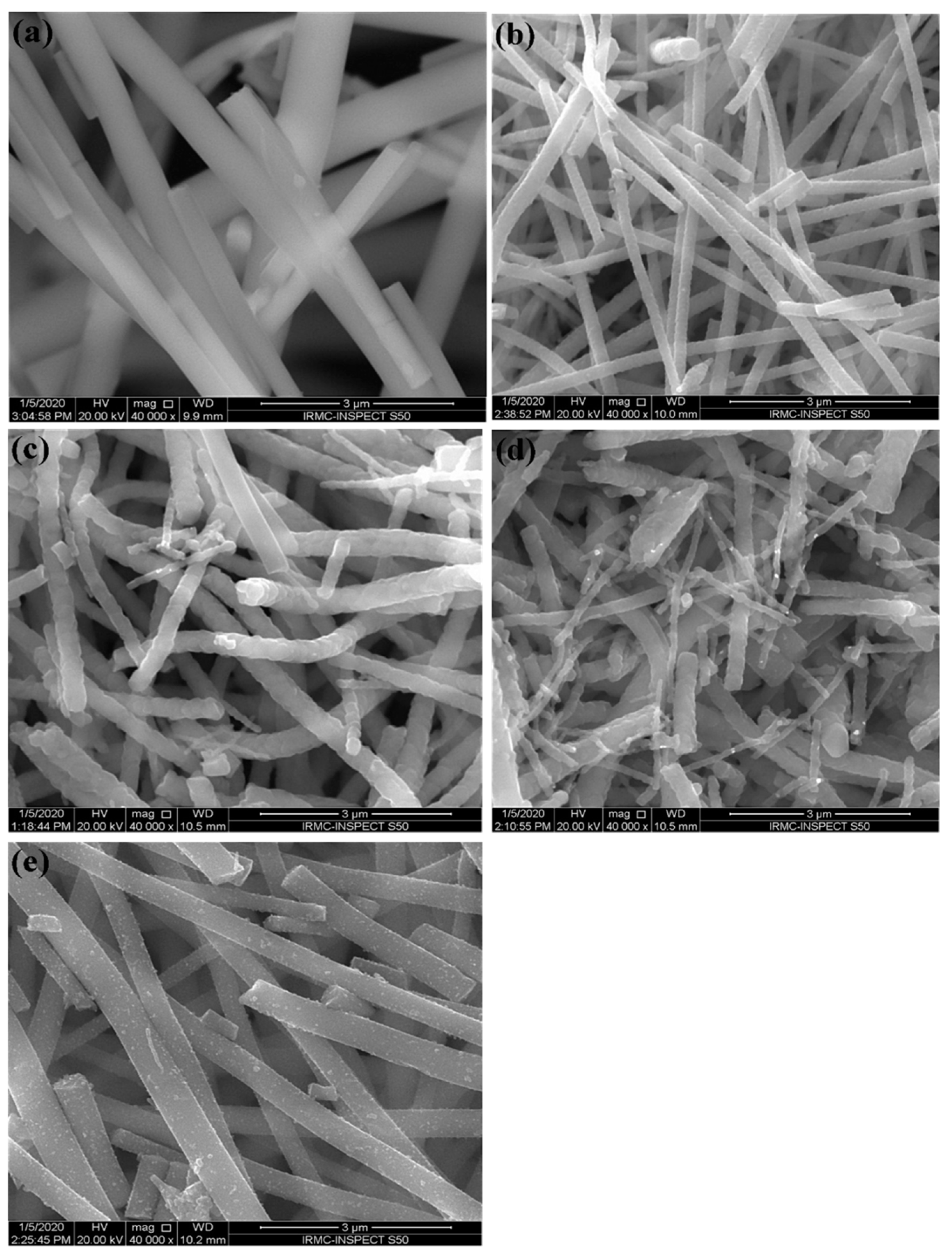
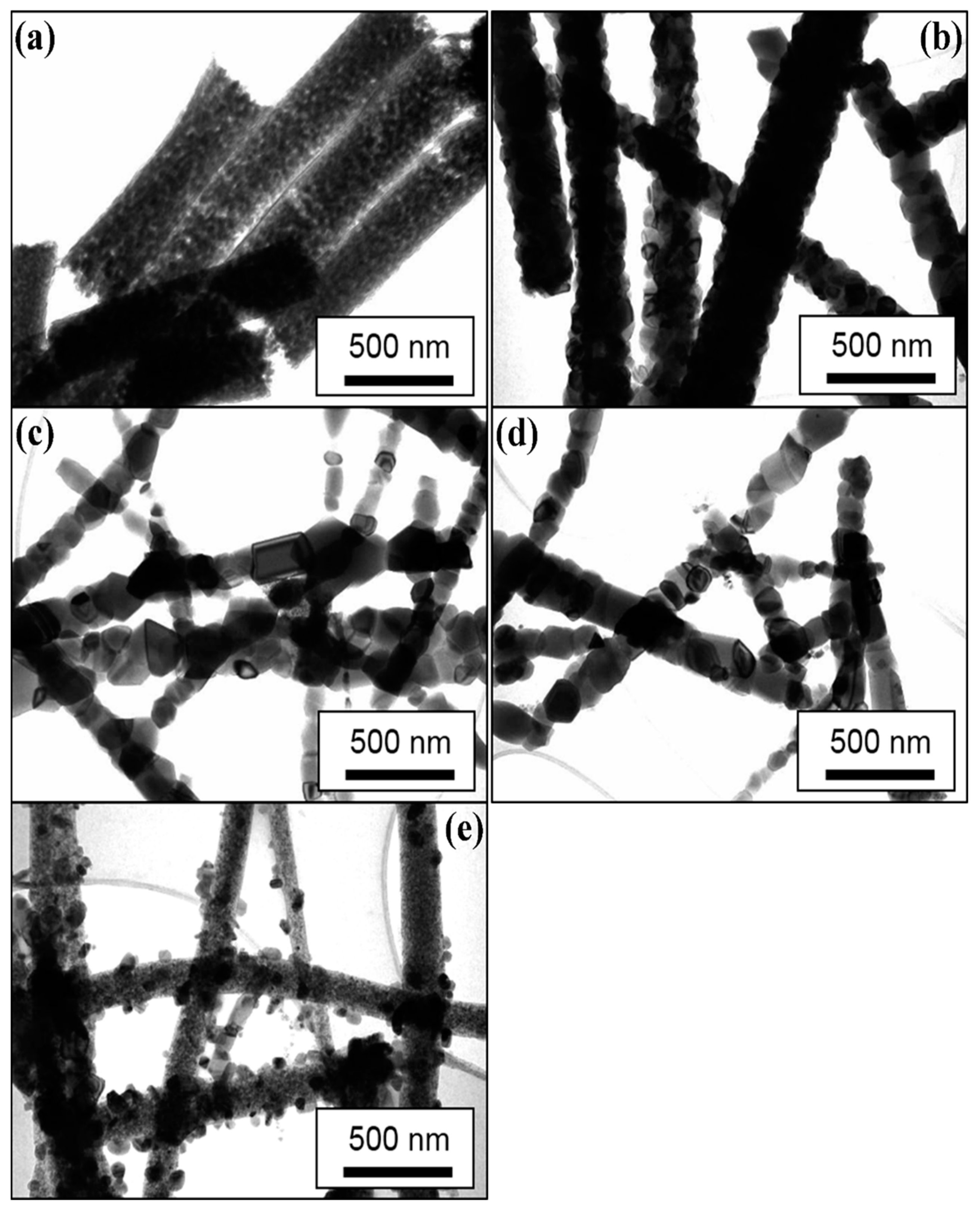
3.2. Microstructure Analysis of the Prepared NFs
3.3. Wide-Band Gap Analysis of Calcined Electrospun TiO2 NFs
3.4. Antibacterial and Antibiofilm Activity of TiO2 NFs
3.4.1. MIC and MBC
3.4.2. Effects of Electrospun TiO2 NFs on the Morphology of Bacterial Cells
3.4.3. Inhibition of Biofilm Formation by TiO2 NFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C: Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A: Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef] [Green Version]
- Kamat, P. Photophysical, Photochemical and Photocatalytic Aspects of Metal Nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Mantravadi, H.B. Effectivity of Titanium Oxide Based Nano Particles on E. coli from Clinical Samples. J. Clin. Diagn. Res. 2017, 11, DC37–DC40. [Google Scholar] [CrossRef]
- Awati, P.; Awate, S.; Shah, P.; Ramaswamy, V. Photocatalytic decomposition of methylene blue using nanocrystalline anatase titania prepared by ultrasonic technique. Catal. Commun. 2003, 4, 393–400. [Google Scholar] [CrossRef]
- Othman, S.; Salam, N.R.A.; Zainal, N.; Basha, R.K.; Talib, R. Antimicrobial Activity of TiO2Nanoparticle-Coated Film for Potential Food Packaging Applications. Int. J. Photoenergy 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Haghi, M.; Hekmatafshar, M.; Janipour, M.B.; Gholizadeh, S.S.; Faraz, M.K.; Sayyadifar, F.; Ghaedi, M. Antibacterial effect of TiO2 nanoparticles on pathogenic strain of E. coli. Int. J. Adv. Biotechnol. Res. 2012, 3, 621–662. [Google Scholar]
- Runa, S.; Khanal, D.; Kemp, M.L.; Payne, C.K. TiO2 Nanoparticles Alter the Expression of Peroxiredoxin Antioxidant Genes. J. Phys. Chem. C 2016, 120, 20736–20742. [Google Scholar] [CrossRef]
- Schmidt, H.; Naumann, M.; Muller, T.; Akarsu, M. Application of spray techniques for new photocatalytic gradient coatings on plastics. Thin Solid Film. 2006, 502, 132–137. [Google Scholar] [CrossRef] [Green Version]
- E Teo, W.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Albetran, H.; Dong, Y.; Low, I.M. Characterization and optimization of electrospun TiO2/PVP nanofibers using Taguchi design of experiment method. J. Asian Ceram. Soc. 2015, 3, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Albetran, H.; Haroosh, H.; Dong, Y.; Prida, V.M.; O’Connor, B.H.; Low, I.M. Phase transformations and crystallization kinetics in electrospun TiO2 nanofibers in air and argon atmospheres. Appl. Phys. A 2014, 116, 161–169. [Google Scholar] [CrossRef]
- Albetran, H.; Low, I.M. Crystallization kinetics and phase transformations in aluminum ion-implanted electrospun TiO2 nanofibers. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Albetran, H.; O’Connor, B.H.; Prida, V.M.; Low, I.M. Effect of vanadium ion implantation on the crystallization kinetics and phase transformation of electrospun TiO2 nanofibers. Appl. Phys. A 2015, 120, 623–634. [Google Scholar] [CrossRef]
- Albetran, H.; Low, I. Parameters controlling the crystallization kinetics of nanostructured TiO2—An overview. Mater. Today Proc. 2019, 16, 25–35. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Xu, S.; Han, G. Fabrication and photocatalytic activity of TiO2 nanofiber membrane. Mater. Lett. 2009, 63, 1761–1763. [Google Scholar] [CrossRef]
- Chandrasekar, R.; Zhang, L.; Howe, J.Y.; Hedin, N.E.; Zhang, Y.; Fong, H. Fabrication and characterization of electrospun titania nanofibers. J. Mater. Sci. 2009, 44, 1198–1205. [Google Scholar] [CrossRef]
- Li, Q.; Satur, D.J.G.; Kim, H.; Kim, H.G. Preparation of sol–gel modified electrospun TiO2 nanofibers for improved photocatalytic decomposition of ethylene. Mater. Lett. 2012, 76, 169–172. [Google Scholar] [CrossRef]
- Xu, S.; Li, A.; Poirier, G.; Yao, N. In Situ Mechanical and Electrical Characterization of Individual TiO2Nanofibers Using a Nanomanipulator System. Scanning 2012, 34, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Sardar, M. TiO2 nanoparticles as an antibacterial agents against E. coli. Int. J. Innov. Res. Sci. Eng. Technol. 2013, 2, 3569–3574. [Google Scholar]
- Albetran, H.; Low, I. Crystallization kinetics study of In-doped and (In-Cr) co-doped TiO2 nanopowders using in-situ high-temperature synchrotron radiation diffraction. Arab. J. Chem. 2020, 13, 3946–3956. [Google Scholar] [CrossRef]
- Albetran, H.; Vega, V.; Prida, V.M.; Low, I.-M. Dynamic Diffraction Studies on the Crystallization, Phase Transformation, and Activation Energies in Anodized Titania Nanotubes. Nanomaterials 2018, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Albetran, H.; Low, I.-M. Effect of indium ion implantation on crystallization kinetics and phase transformation of anodized titania nanotubes using in-situ high-temperature radiation diffraction. J. Mater. Res. 2016, 31, 1588–1595. [Google Scholar] [CrossRef]
- Ravishankar, R.V.; Jamuna, B.A. Nanoparticles and their potential application as antimicrobials. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Mendez-Vilas, A., Ed.; University of Mysore: Mysore, India, 2011; pp. 197–209. [Google Scholar]
- Arora, H.; Doty, C.; Yuan, Y.; Boyle, J.; Petras, K.; Rabatic, B.; Paunesku, T.; Woloschak, G.E. Titanium Dioxide Nanocomposites. In Nanomaterials for Life Sciences; Kumar, C.S.S.R., Ed.; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, Germany, 2010; pp. 1–42. [Google Scholar]
- Albetran, H.; O’Connor, B.; Low, I.-M. Effect of calcination on band gaps for electrospun titania nanofibers heated in air–argon mixtures. Mater. Des. 2016, 92, 480–485. [Google Scholar] [CrossRef] [Green Version]
- Bhadwal, A.S.; Tripathi, R.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484. [Google Scholar] [CrossRef]
- Balasamy, R.J.; Ravinayagam, V.; Alomari, M.; Ansari, M.A.; Almofty, S.A.; Rehman, S.; Dafalla, H.; Marimuthu, P.R.; Akhtar, S.; Hamad, M. Cisplatin delivery, anticancer and antibacterial properties of Fe/SBA-16/ZIF-8 nanocomposite. RSC Adv. 2019, 9, 42395–42408. [Google Scholar] [CrossRef] [Green Version]
- Sultan, A.; Khan, H.M.; Malik, A.; Ansari, A.; Azam, A.; Perween, N. Antibacterial activity of ZnO nanoparticles against ESBL and Amp-C producing gram negative isolates from superficial wound infections. Int. J. Curr. Microbiol. App. Sci. 2015, 1, 38–47. [Google Scholar]
- Baig, U.; Ansari, M.A.; Gondal, M.; Akhtard, S.; Khan, F.A.; Falathaf, W.S. Single step production of high-purity copper oxide-titanium dioxide nanocomposites and their effective antibacterial and anti-biofilm activity against drug-resistant bacteria. Mater. Sci. Eng. C 2020, 113, 110992. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.A.A.; Alam, J.; Shukla, A.K.; Alhoshan, M.; Ansari, M.A.; Al-Masry, W.A.; Rehman, S.; Alam, M. Evaluation of antibacterial and antifouling properties of silver-loaded GO polysulfone nanocomposite membrane against Escherichia coli, Staphylococcus aureus, and BSA protein. React. Funct. Polym. 2019, 140, 136–147. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Alam, M.; Kaushik, A. Selective ion removal and antibacterial activity of silver-doped multi-walled carbon nanotube/polyphenylsulfone nanocomposite membranes. Mater. Chem. Phys. 2019, 233, 102–112. [Google Scholar] [CrossRef]
- Gamboa, J.A.; Pasquevich, D.M. Effect of Chlorine Atmosphere on the Anatase-Rutile Transformation. J. Am. Ceram. Soc. 1992, 75, 2934–2938. [Google Scholar] [CrossRef]
- Szilágyi, I.M.; Santala, E.; Heikkilä, M.; Pore, V.; Kemell, M.; Nikitin, T.; Teucher, G.; Firkala, T.; Khriachtchev, L.; Rasanen, M.; et al. Photocatalytic properties of WO3/TiO2 core/shell nanofibers prepared by electrospinning and atomic layer deposition. Chem. Vap. Depos. 2013, 19, 149–155. [Google Scholar] [CrossRef]
- Albetran, H.; O’Connor, B.H.; Low, I.-M. Activation energies for phase transformations in electrospun titania nanofibers: Comparing the influence of argon and air atmospheres. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Natoli, A.; Cabeza, A.; Torre, Ángeles, G.; Aranda, M.A.G.; Santacruz, I.; Cabeza, A. Colloidal Processing of Macroporous TiO2 Materials for Photocatalytic Water Treatment. J. Am. Ceram. Soc. 2011, 95, 502–508. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A: Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Seo, H.; Baker, L.R.; Hervier, A.; Kim, J.; Whitten, J.L.; Somorjai, G.A. Generation of Highly n-Type Titanium Oxide Using Plasma Fluorine Insertion. Nano Lett. 2011, 11, 751–756. [Google Scholar] [CrossRef]
- Andersson, S.; Collén, B.; Kuylenstierna, U.; Magnéli, A.; Pestmalis, H.; Åsbrink, S. Phase Analysis Studies on the Titanium-Oxygen System. Acta Chem. Scand. 1957, 11, 1641–1652. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Nagalakshmi, M.; Karthikeyan, C.; Anusuya, N.; Brundha, C.; Basu, M.J.; Karuppuchamy, S. Synthesis of TiO2 nanofiber for photocatalytic and antibacterial applications. J. Mater. Sci. Mater. Electron. 2017, 28, 15915–15920. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vardanyan, Z.; Gevorkyan, V.; Ananyan, M.; Vardapetyan, H.; Trchounian, A. Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport. J. Nanobiotechnol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pigeot-Remy, S.; Simonet, F.; Errazuriz-Cerda, E.; Lazzaroni, J.; Atlan, D.; Guillard, C. Photocatalysis and disinfection of water: Identification of potential bacterial targets. Appl. Catal. B: Environ. 2011, 104, 390–398. [Google Scholar] [CrossRef]
- Ranjan, S.; Ramalingam, C. Titanium dioxide nanoparticles induce bacterial membrane rupture by reactive oxygen species generation. Environ. Chem. Lett. 2016, 14, 487–494. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, L.; Ma, H.; Zhang, H.; Guo, L.-H. Quantitative Analysis of Reactive Oxygen Species Photogenerated on Metal Oxide Nanoparticles and Their Bacteria Toxicity: The Role of Superoxide Radicals. Environ. Sci. Technol. 2017, 51, 10137–10145. [Google Scholar] [CrossRef]
- Chakra, C.S.; Mateti, S. Structural, Antimicrobial and Electrochemical Properties of Cu/TiO2 Nanocomposites. J. Nanosci. Technol. 2018, 4, 331–334. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef] [Green Version]
- Senarathna, U.L.N.H.; Fernando, N.; Gunasekara, C.; Weerasekera, M.M.; Hewageegana, H.G.S.P.; Arachchi, N.D.; Siriwardena, H.D.; Jayaweera, P.M. Enhanced antibacterial activity of TiO2 nanoparticle surface modified with Garcinia zeylanica extract. Chem. Cent. J. 2017, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Park, Y.-S.; Cho, Y.-K. Significantly enhanced antibacterial activity of TiO 2 nanofibers with hierarchical nanostructures and controlled crystallinity. Analyst 2015, 140, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N.; Valadbeigi, T.; Valadbaeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef]
- Santhosh, S.M.; Natarajan, K. Antibiofilm Activity of Epoxy/Ag-TiO2 Polymer Nanocomposite Coatings against Staphylococcus Aureus and Escherichia Coli. Coatings 2015, 5, 95–114. [Google Scholar] [CrossRef]






| Calcination Conditions | Eg (eV) |
|---|---|
| As-electrospun | 3.33 |
| 100% Air | 3.09 |
| 50% Air and 50% Argon | 2.94 |
| 25% Air and75% Argon | 2.91 |
| 100% Argon | 2.18 |
| Electrospun TiO2 Nanofibers Code | Calcination Conditions | Methicillin Resistant S. aureus | P. aeruginosa | ||
|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | ||
| (a) | 100% Air | 7 | 14 | 7 | 14 |
| (b) | 50% Air and 50% Argon | 7 | 14 | 7 | 14 |
| (c) | 25% Air and 75% Argon | 6 | 12 | 3 | 6 |
| (d) | 100% Argon | >16 | >32 | >16 | >32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.A.; Albetran, H.M.; Alheshibri, M.H.; Timoumi, A.; Algarou, N.A.; Akhtar, S.; Slimani, Y.; Almessiere, M.A.; Alahmari, F.S.; Baykal, A.; et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics 2020, 9, 572. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090572
Ansari MA, Albetran HM, Alheshibri MH, Timoumi A, Algarou NA, Akhtar S, Slimani Y, Almessiere MA, Alahmari FS, Baykal A, et al. Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria. Antibiotics. 2020; 9(9):572. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090572
Chicago/Turabian StyleAnsari, Mohammad Azam, Hani Manssor Albetran, Muidh Hamed Alheshibri, Abdelmajid Timoumi, Norah Abdullah Algarou, Sultan Akhtar, Yassine Slimani, Munirah Abdullah Almessiere, Fatimah Saad Alahmari, Abdulhadi Baykal, and et al. 2020. "Synthesis of Electrospun TiO2 Nanofibers and Characterization of Their Antibacterial and Antibiofilm Potential against Gram-Positive and Gram-Negative Bacteria" Antibiotics 9, no. 9: 572. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090572







