Overcoming Intrinsic and Acquired Resistance Mechanisms Associated with the Cell Wall of Gram-Negative Bacteria
Abstract
:1. Introduction
2. Outer Membrane
3. Peptidoglycan Layer and Inner Membrane
4. Porins
5. Efflux Pumps
6. Combinatorial Approaches
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Pattnaik, D.; Panda, S.S.; Singh, N.; Sahoo, S.; Mohapatra, I.; Jena, J. Multidrug resistant, extensively drug resistant and pan drug resistant Gram-negative bacteria at a tertiary care centre in Bhubaneswar. Int. J. Community Med. Public Health 2019, 6, 567–572. [Google Scholar] [CrossRef]
- De Rosa, A.; Mutters, N.T.; Mastroianni, C.M.; Kaiser, S.J.; Günther, F. Distribution of carbapenem resistance mechanisms in clinical isolates of XDR Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Nichols, L. Death from pan-resistant superbug. Autops. Case Rep. 2019, 9, e2019106. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, CDC; 2019.
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [Green Version]
- Delcour, A.H. Outer membrane permeability and antibiotic resistance. Biochim. Biophys. Acta 2009, 1794, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Schurek, K.N.; Marr, A.K.; Taylor, P.K.; Wiegand, I.; Semenec, L.; Khaira, B.K.; Hancock, R.E.W. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 4213–4219. [Google Scholar] [CrossRef] [Green Version]
- Zgurskaya, H.I.; Löpez, C.A.; Gnanakaran, S. Permeability barrier of Gram-negative cell envelopes and approaches to bypass it. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, M.-J.; Ko, K.S. Mutant prevention concentrations of colistin for Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae clinical isolates. J. Antimicrob. Chemother. 2014, 69, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.S.; Lim, K.B.; Krueger, J.; Kim, K.; Guo, L.; Hackett, M.; Miller, S.I. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 1998, 27, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.A.; Herrera, C.M.; Fernandez, L.; Hankins, J.V.; Trent, M.S.; Hancock, R.E.W. The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob. Agents Chemother. 2011, 55, 3743–3751. [Google Scholar] [CrossRef] [Green Version]
- Beceiro, A.; Llobet, E.; Aranda, J.; Bengoechea, J.A.; Doumith, M.; Hornsey, M.; Dhanji, H.; Chart, H.; Bou, G.; Livermore, D.M.; et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob. Agents Chemother. 2011, 55, 3370–3379. [Google Scholar] [CrossRef] [Green Version]
- Macfarlane, E.L.A.; Kwasnicka, A.; Hancock, R.E.W. Role of Pseudomonas aeruginosa PhoP-PhoQ in resistance to antimicrobial cationic peptides and aminoglycosides. Microbiology 2000, 146, 2543–2554. [Google Scholar] [CrossRef] [Green Version]
- Gunn, J.S.; Miller, S.I. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J. Bacteriol. 1996, 178, 6857–6864. [Google Scholar] [CrossRef] [Green Version]
- Wand, M.E.; Sutton, J.M. Mutations in the two component regulator systems PmrAB and PhoPQ give rise to increased colistin resistance in Citrobacter and Enterobacter spp. J. Med. Microbiol. 2020, 69, 521–529. [Google Scholar] [CrossRef]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [Green Version]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef] [Green Version]
- Marceau, M.; Sebbane, F.; Ewann, F.; Collyn, F.; Lindner, B.; Campos, M.A.; Bengoechea, J.-A.; Simonet, M. The pmrF polymyxin-resistance operon of Yersinia pseudotuberculosis is upregulated by the PhoP–PhoQ two-component system but not by PmrA–PmrB, and is not required for virulence. Microbiology 2004, 150, 3947–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, E.X.; Wozniak, J.E.; Weiss, D.S. Methods to evaluate colistin heteroresistance in Acinetobacter baumannii. Methods Mol. Biol. 2019, 1946, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Weiss, D.S. Heteroresistance: A cause of unexplained antibiotic treatment failure? PLoS Pathog. 2019, 15, e1007726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Rayner, C.R.; Nation, R.L.; Owen, R.J.; Spelman, D.; Tan, K.E.; Liolios, L. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2006, 50, 2946–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayol, A.; Nordmann, P.; Brink, A.; Poirel, L. Heteroresistance to Colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob. Agents Chemother. 2015, 59, 2780–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Xu, C.; Fang, R.; Cao, J.; Zhang, X.; Zhao, Y.; Dong, G.; Sun, Y.; Zhou, T. Resistance and heteroresistance to colistin in Pseudomonas aeruginosa isolates from Wenzhou, China. Antimicrob. Agents Chemother. 2019, 63, e00556-19. [Google Scholar] [CrossRef] [Green Version]
- Guérin, F.; Isnard, C.; Sinel, C.; Morand, P.; Dhalluin, A.; Cattoir, V.; Giard, J.-C. Cluster-dependent colistin hetero-resistance in Enterobacter cloacae complex. J. Antimicrob. Chemother. 2016, 71, 3058–3061. [Google Scholar] [CrossRef] [Green Version]
- Hjort, K.; Nicoloff, H.; Andersson, D.I. Unstable tandem gene amplification generates heteroresistance (variation in resistance within a population) to colistin in Salmonella enterica. Mol. Microbiol. 2016, 102, 274–289. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Doumith, M.; Godbole, G.; Ashton, P.; Larkin, L.; Dallman, T.; Day, M.; Day, M.; Muller-Pebody, B.; Ellington, M.J.; de Pinna, E.; et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J. Antimicrob. Chemother. 2016, 71, 2300–2305. [Google Scholar] [CrossRef] [Green Version]
- Moffatt, J.H.; Harper, M.; Harrison, P.; Hale, J.D.F.; Vinogradov, E.; Seemann, T.; Henry, R.; Crane, B.; St Michael, F.; Cox, A.D.; et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob. Agents Chemother. 2010, 54, 4971–4977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.L.; Bains, M.; Bell, A.; Hancock, R.E. Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: Isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob. Agents Chemother. 1992, 36, 2566–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Y.-J.; Sun, H.-R.; Luo, X.-W.; Liu, J.-H.; Pan, Y.-S.; Wu, H.; Yuan, L.; Liang, J.; He, D.-D.; Hu, G.-Z. CpxR regulates the colistin susceptibility of Salmonella Typhimurium by a multitarget mechanism. J. Antimicrob. Chemother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Plésiat, P.; Jeannot, K. A two-component regulatory system interconnects resistance to polymyxins, aminoglycosides, fluoroquinolones, and β-lactams in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2011, 55, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- Telke, A.A.; Olaitan, A.O.; Morand, S.; Rolain, J.-M. soxRS induces colistin hetero-resistance in Enterobacter asburiae and Enterobacter cloacae by regulating the acrAB-tolC efflux pump. J. Antimicrob. Chemother. 2017, 72, 2715–2721. [Google Scholar] [CrossRef]
- Shinohara, D.R.; Menegucci, T.C.; Fedrigo, N.H.; Migliorini, L.B.; Carrara-Marroni, F.E.; Maria dos Anjos, M.; Cardoso, C.L.; Nishiyama, S.A.B.; Tognim, M.C.B. Synergistic activity of polymyxin B combined with vancomycin against carbapenem-resistant and polymyxin-resistant Acinetobacter baumannii: First in vitro study. J. Med. Microbiol. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Bulman, Z.P.; Tsuji, B.T.; Kaye, K.S. Shifting gears: The future of polymyxin antibiotics. Antibiotics 2019, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Velkov, T.; Roberts, K.D.; Thompson, P.E.; Li, J. Polymyxins: A new hope in combating Gram-negative superbugs? Future Med. Chem. 2016, 8, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Ofek, I.; Cohen, S.; Rahmani, R.; Kabha, K.; Tamarkin, D.; Herzig, Y.; Rubinstein, E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental Gram-negative infections in mice. Antimicrob. Agents Chemother. 1994, 38, 374–377. [Google Scholar] [CrossRef] [Green Version]
- Vaara, M. Polymyxin derivatives that sensitize Gram-negative bacteria to other antibiotics. Molecules 2019, 24, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danner, R.L.; Joiner, K.A.; Rubin, M.; Patterson, W.H.; Johnson, N.; Ayers, K.M.; Parrillo, J.E. Purification, toxicity, and antiendotoxin activity of polymyxin B nonapeptide. Antimicrob. Agents Chemother. 1989, 33, 1428–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zurawski, D.V.; Reinhart, A.A.; Alamneh, Y.A.; Pucci, M.J.; Si, Y.; Abu-Taleb, R.; Shearer, J.P.; Demons, S.T.; Tyner, S.D.; Lister, T. SPR741, an antibiotic adjuvant, potentiates the in vitro and in vivo activity of rifampin against clinically relevant extensively drug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2017, 61, e01239-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spero Reports Preliminary Findings from Phase 1 Clinical Trial of SPR206 and Plans to Advance Program with Alliance Partners Everest Medicines and the Department of Defense. Available online: https://investors.sperotherapeutics.com/news-releases/news-release-details/spero-reports-preliminary-findings-phase-1-clinical-trial-spr206 (accessed on 21 April 2020).
- Pitt, M.E.; Cao, M.D.; Butler, M.S.; Ramu, S.; Ganesamoorthy, D.; Blaskovich, M.A.T.; Coin, L.J.M.; Cooper, M.A. Octapeptin C4 and polymyxin resistance occur via distinct pathways in an epidemic XDR Klebsiella pneumoniae ST258 isolate. J. Antimicrob. Chemother. 2019, 74, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Gallardo-Godoy, A.; Swarbrick, J.D.; Blaskovich, M.A.; Elliott, A.G.; Han, M.; Thompson, P.E.; Roberts, K.D.; Huang, J.X.; Becker, B.; et al. Structure, function, and biosynthetic origin of octapeptin antibiotics active against extensively drug-resistant Gram-negative bacteria. Cell Chem. Biol. 2018, 25, 380–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaskovich, M.A.T.; Pitt, M.E.; Elliott, A.G.; Cooper, M.A. Can octapeptin antibiotics combat extensively drug-resistant (XDR) bacteria? Expert Rev. Anti-Infect Ther. 2018, 16, 485–499. [Google Scholar] [CrossRef] [PubMed]
- CARB-X Funds University of Queensland to Accelerate the Development of a New Class of Last-Resort Antibiotics to Treat Deadly Superbug Infections. Carb-X. Available online: https://carb-x.org/carb-x-news/carb-x-funds-university-of-queensland-to-accelerate-the-development-of-a-new-class-of-last-resort-antibiotics-to-treat-deadly-superbug-infections (accessed on 8 May 2020).
- Tsai, C.N.; MacNair, C.R.; Cao, M.P.T.; Perry, J.N.; Magolan, J.; Brown, E.D.; Coombes, B.K. Targeting two-component systems uncovers a small-molecule inhibitor of Salmonella virulence. Cell Chem. Biol. 2020, 27, 793–805. [Google Scholar] [CrossRef]
- Erwin, A.L. Antibacterial drug discovery targeting the lipopolysaccharide biosynthetic enzyme LpxC. Cold Spring Harb. Perspect. Med. 2016, 6, a025304. [Google Scholar] [CrossRef]
- Tomaras, A.P.; McPherson, C.J.; Kuhn, M.; Carifa, A.; Mullins, L.; George, D.; Desbonnet, C.; Eidem, T.M.; Montgomery, J.I.; Brown, M.F.; et al. LpxC inhibitors as new antibacterial agents and tools for studying regulation of lipid A biosynthesis in Gram-negative pathogens. mBio 2014, 5, e01551-14. [Google Scholar] [CrossRef] [Green Version]
- Krause, K.M.; Haglund, C.M.; Hebner, C.; Serio, A.W.; Lee, G.; Nieto, V.; Cohen, F.; Kane, T.R.; Machajewski, T.D.; Hildebrandt, D.; et al. Potent LpxC inhibitors with in vitro activity against multidrug-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 63, e00977-19. [Google Scholar] [CrossRef]
- A Study to Assess the Safety, Tolerability, and Pharmacokinetics of ACHN-975 in Healthy Volunteers—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01597947 (accessed on 29 April 2020).
- Cohen, F.; Aggen, J.B.; Andrews, L.D.; Assar, Z.; Boggs, J.; Choi, T.; Dozzo, P.; Easterday, A.N.; Haglund, C.M.; Hildebrandt, D.J.; et al. Optimization of LpxC inhibitors for antibacterial activity and cardiovascular safety. ChemMedChem 2019, 14, 1560–1572. [Google Scholar] [CrossRef]
- García-Quintanilla, M.; Carretero-Ledesma, M.; Moreno-Martínez, P.; Martín-Peña, R.; Pachón, J.; McConnell, M.J. Lipopolysaccharide loss produces partial colistin dependence and collateral sensitivity to azithromycin, rifampicin and vancomycin in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2015, 46, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Retention of virulence following adaptation to colistin in Acinetobacter baumannii reflects the mechanism of resistance. J. Antimicrob. Chemother. 2015, 70, 2209–2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Quintanilla, M.; Caro-Vega, J.M.; Pulido, M.R.; Moreno-Martínez, P.; Pachón, J.; McConnell, M.J. Inhibition of LpxC increases antibiotic susceptibility in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 5076–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werneburg, M.; Zerbe, K.; Juhas, M.; Bigler, L.; Stalder, U.; Kaech, A.; Ziegler, U.; Obrecht, D.; Eberl, L.; Robinson, J.A. Inhibition of lipopolysaccharide transport to the outer membrane in Pseudomonas aeruginosa by peptidomimetic antibiotics. ChemBioChem 2012, 13, 1767–1775. [Google Scholar] [CrossRef] [Green Version]
- Vetterli, S.U.; Moehle, K.; Robinson, J.A. Synthesis and antimicrobial activity against Pseudomonas aeruginosa of macrocyclic β-hairpin peptidomimetic antibiotics containing N-methylated amino acids. Bioorg. Med. Chem. 2016, 24, 6332–6339. [Google Scholar] [CrossRef] [Green Version]
- Martin-Loeches, I.; Dale, G.E.; Torres, A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti-Infect. Ther. 2018, 16, 259–268. [Google Scholar] [CrossRef]
- Provenzani, A.; Hospodar, A.R.; Meyer, A.L.; Leonardi Vinci, D.; Hwang, E.Y.; Butrus, C.M.; Polidori, P. Multidrug-resistant gram-negative organisms: A review of recently approved antibiotics and novel pipeline agents. Int. J. Clin. Pharm. 2020, 42, 1016–1025. [Google Scholar] [CrossRef]
- Harrison, L.B.; Fowler, R.C.; Abdalhamid, B.; Selmecki, A.; Hanson, N.D. lptG contributes to changes in membrane permeability and the emergence of multidrug hypersusceptibility in a cystic fibrosis isolate of Pseudomonas aeruginosa. Microbiol. Open 2019, 8, e844. [Google Scholar] [CrossRef] [Green Version]
- Clairfeuille, T.; Buchholz, K.R.; Li, Q.; Verschueren, E.; Liu, P.; Sangaraju, D.; Park, S.; Noland, C.L.; Storek, K.M.; Nickerson, N.N.; et al. Structure of the essential inner membrane lipopolysaccharide–PbgA complex. Nature 2020, 584, 479–483. [Google Scholar] [CrossRef]
- Nickerson, N.N.; Jao, C.C.; Xu, Y.; Quinn, J.; Skippington, E.; Alexander, M.K.; Miu, A.; Skelton, N.; Hankins, J.V.; Lopez, M.S.; et al. A novel inhibitor of the LolCDE ABC transporter essential for lipoprotein trafficking in Gram-negative bacteria. Antimicrob. Agents Chemother. 2018, 62, e02151-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrivastava, R.; Chng, S.-S. Lipid trafficking across the Gram-negative cell envelope. J. Biol. Chem. 2019, 294, 14175–14184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, A.G.; Huang, J.X.; Neve, S.; Zuegg, J.; Edwards, I.A.; Cain, A.K.; Boinett, C.J.; Barquist, L.; Lundberg, C.V.; Steen, J.; et al. An amphipathic peptide with antibiotic activity against multidrug-resistant Gram-negative bacteria. Nat. Commun. 2020, 11, 3184. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Gil, T.; Molina, R.; Alcorlo, M.; Hermoso, J.A. Renew or die: The molecular mechanisms of peptidoglycan recycling and antibiotic resistance in Gram-negative pathogens. Drug Resist. Update 2016, 28, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Impey, R.E.; Lee, M.; Hawkins, D.A.; Sutton, J.M.; Panjikar, S.; Perugini, M.A.; Soares da Costa, T.P. Mis-annotations of a promising antibiotic target in high-priority gram-negative pathogens. FEBS Lett. 2020, 594, 1453–1463. [Google Scholar] [CrossRef]
- Impey, R.E.; Panjikar, S.; Hall, C.J.; Bock, L.J.; Sutton, J.M.; Perugini, M.A.; Soares da Costa, T.P. Identification of two dihydrodipicolinate synthase isoforms from Pseudomonas aeruginosa that differ in allosteric regulation. FEBS J. 2020, 287, 386–400. [Google Scholar] [CrossRef]
- Soares da Costa, T.P.; Christensen, J.B.; Desbois, S.; Gordon, S.E.; Gupta, R.; Hogan, C.J.; Nelson, T.G.; Downton, M.T.; Gardhi, C.K.; Abbott, B.M.; et al. Chapter Nine—Quaternary Structure Analyses of an Essential Oligomeric Enzyme. In Methods in Enzymology; Cole, J.L., Ed.; Analytical Ultracentrifugation; Academic Press: Cambridge, MA, USA, 2015; Volume 562, pp. 205–223. [Google Scholar]
- Christoff, R.M.; Gardhi, C.K.; da Costa, T.P.S.; Perugini, M.A.; Abbott, B.M. Pursuing DHDPS: An enzyme of unrealised potential as a novel antibacterial target. Med. Chem. Commun. 2019, 10, 1581–1588. [Google Scholar] [CrossRef]
- Soares da Costa, T.P.; Desbois, S.; Dogovski, C.; Gorman, M.A.; Ketaren, N.E.; Paxman, J.J.; Siddiqui, T.; Zammit, L.M.; Abbott, B.M.; Robins-Browne, R.M.; et al. Structural determinants defining the allosteric inhibition of an essential antibiotic target. Structure 2016, 24, 1282–1291. [Google Scholar] [CrossRef] [Green Version]
- Hutton, C.A.; Perugini, M.A.; Gerrard, J.A. Inhibition of lysine biosynthesis: An evolving antibiotic strategy. Mol. Biosyst. 2007, 3, 458–465. [Google Scholar] [CrossRef]
- Christensen, J.B.; Soares da Costa, T.P.; Faou, P.; Pearce, F.G.; Panjikar, S.; Perugini, M.A. Structure and function of cyanobacterial DHDPS and DHDPR. Sci. Rep. 2016, 6, 37111. [Google Scholar] [CrossRef]
- Gupta, R.; Soares da Costa, T.P.; Faou, P.; Dogovski, C.; Perugini, M.A. Comparison of untagged and his-tagged dihydrodipicolinate synthase from the enteric pathogen Vibrio cholerae. Protein Expr. Purif. 2018, 145, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Eveland, S.S.; Onishi, H.R.; Pompliano, D.L. Kinetic mechanism of the Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme: Use of a glutathione s-transferase fusion. Biochemistry 1996, 35, 16264–16269. [Google Scholar] [CrossRef] [PubMed]
- Impey, R.E.; Soares da Costa, T.P. Review: Targeting the biosynthesis and incorporation of amino acids into peptidoglycan as an antibiotic approach against Gram negative bacteria. EC Microbiol. 2018, 14, 200–209. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The revival of polymyxins for the management of multidrug-resistant Gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Hancock, R.E.W. Adaptive and mutational resistance: Role of porins and efflux pumps in drug resistance. Clin. Microbiol. Rev. 2012, 25, 661–681. [Google Scholar] [CrossRef] [Green Version]
- Vergalli, J.; Bodrenko, I.V.; Masi, M.; Moynié, L.; Acosta-Gutiérrez, S.; Naismith, J.H.; Davin-Regli, A.; Ceccarelli, M.; van den Berg, B.; Winterhalter, M.; et al. Porins and small-molecule translocation across the outer membrane of Gram-negative bacteria. Nat. Rev. Microbiol. 2020, 18, 164–176. [Google Scholar] [CrossRef]
- Choi, U.; Lee, C.-R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front. Microbiol. 2019, 10, 953. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Wozniak, A.; Villagra, N.A.; Undabarrena, A.; Gallardo, N.; Keller, N.; Moraga, M.; Román, J.C.; Mora, G.C.; García, P. Porin alterations present in non-carbapenemase-producing Enterobacteriaceae with high and intermediate levels of carbapenem resistance in Chile. J. Med. Microbiol. 2012, 61, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- García-Fernández, A.; Miriagou, V.; Papagiannitsis, C.C.; Giordano, A.; Venditti, M.; Mancini, C.; Carattoli, A. An ertapenem-resistant extended-spectrum-β-lactamase-producing Klebsiella pneumoniae clone carries a novel OmpK36 porin variant. Antimicrob. Agents Chemother. 2010, 54, 4178–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikaido, H.; Nikaido, K.; Harayama, S. Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem. 1991, 266, 770–779. [Google Scholar] [PubMed]
- Bellido, F.; Martin, N.L.; Siehnel, R.J.; Hancock, R.E. Reevaluation, using intact cells, of the exclusion limit and role of porin OprF in Pseudomonas aeruginosa outer membrane permeability. J. Bacteriol. 1992, 174, 5196–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kos, V.N.; McLaughlin, R.E.; Gardner, H.A. Identification of unique in-frame deletions in OprD among clinical isolates of Pseudomonas aeruginosa. Pathog. Dis. 2016, 74, ftw031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Heijden, J.; Reynolds, L.A.; Deng, W.; Mills, A.; Scholz, R.; Imami, K.; Foster, L.J.; Duong, F.; Finlay, B.B. Salmonella rapidly regulates membrane permeability to survive oxidative stress. mBio 2016, 7, e01238-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Prasadarao, N.V. Outer membrane protein A and OprF: Versatile roles in Gram-negative bacterial infections. FEBS J. 2012, 279, 919–931. [Google Scholar] [CrossRef]
- Woodruff, W.A.; Hancock, R.E. Pseudomonas aeruginosa outer membrane protein F: Structural role and relationship to the Escherichia coli OmpA protein. J. Bacteriol. 1989, 171, 3304–3309. [Google Scholar] [CrossRef] [Green Version]
- De Mot, R.; Vanderleyden, J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both Gram-positive and Gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 1994, 12, 333–334. [Google Scholar] [CrossRef]
- Bouffartigues, E.; Moscoso, J.A.; Duchesne, R.; Rosay, T.; Fito-Boncompte, L.; Gicquel, G.; Maillot, O.; Bénard, M.; Bazire, A.; Brenner-Weiss, G.; et al. The absence of the Pseudomonas aeruginosa OprF protein leads to increased biofilm formation through variation in c-di-GMP level. Front. Microbiol. 2015, 6, 630. [Google Scholar] [CrossRef] [Green Version]
- Vila-Farrés, X.; Parra-Millán, R.; Sánchez-Encinales, V.; Varese, M.; Ayerbe-Algaba, R.; Bayó, N.; Guardiola, S.; Pachón-Ibáñez, M.E.; Kotev, M.; García, J.; et al. Combating virulence of Gram-negative bacilli by OmpA inhibition. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef] [Green Version]
- Hagan, C.L.; Wzorek, J.S.; Kahne, D. Inhibition of the β-barrel assembly machine by a peptide that binds BamD. Proc. Natl. Acad. Sci. USA 2015, 112, 2011–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bambeke, F.; Michot, J.-M.; Tulkens, P.M. Antibiotic efflux pumps in eukaryotic cells: Occurrence and impact on antibiotic cellular pharmacokinetics, pharmacodynamics and toxicodynamics. J. Antimicrob. Chemother. 2003, 51, 1067–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bambeke, F.; Glupczynski, Y.; Plésiat, P.; Pechère, J.C.; Tulkens, P.M. Antibiotic efflux pumps in prokaryotic cells: Occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J. Antimicrob. Chemother. 2003, 51, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venter, H.; Mowla, R.; Ohene-Agyei, T.; Ma, S. RND-type drug efflux pumps from Gram-negative bacteria: Molecular mechanism and inhibition. Front. Microbiol. 2015, 6, 377. [Google Scholar] [CrossRef]
- Marquez, B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie 2005, 87, 1137–1147. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Neuberger, A.; Du, D.; Luisi, B.F. Structure and mechanism of bacterial tripartite efflux pumps. Res. Microbiol. 2018, 169, 401–413. [Google Scholar] [CrossRef]
- Lubelski, J.; Konings, W.N.; Driessen, A.J.M. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol. Mol. Biol. Rev. 2007, 71, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Weston, N.; Sharma, P.; Ricci, V.; Piddock, L.J.V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 2018, 169, 425–431. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Lee, A.; Hoshino, K.; Ishida, H.; Mistry, A.; Warren, M.S.; Boyer, E.; Chamberland, S.; Lee, V.J. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1999, 43, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, Y.; Komori, Y.; Mima, T.; Kuroda, T.; Mizushima, T.; Tsuchiya, T. Construction of a series of mutants lacking all of the four major mex operons for multidrug efflux pumps or possessing each one of the operons from Pseudomonas aeruginosa PAO1: MexCD-OprJ is an inducible pump. FEMS Microbiol. Lett. 2001, 202, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreier, J.; Ruggerone, P. Interaction of antibacterial compounds with RND efflux pumps in Pseudomonas aeruginosa. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, K.; Latifi, T.; Groisman, E.A. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2006, 59, 126–141. [Google Scholar] [CrossRef]
- Kobayashi, N.; Nishino, K.; Yamaguchi, A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 2001, 183, 5639–5644. [Google Scholar] [CrossRef] [Green Version]
- Iyer, R.; Moussa, S.H.; Tommasi, R.; Miller, A.A. Role of the Klebsiella pneumoniae TolC porin in antibiotic efflux. Res. Microbiol. 2019, 170, 112–116. [Google Scholar] [CrossRef]
- Hansen, L.H.; Jensen, L.B.; Sørensen, H.I.; Sørensen, S.J. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. J. Antimicrob. Chemother. 2007, 60, 145–147. [Google Scholar] [CrossRef]
- Guérin, F.; Lallement, C.; Isnard, C.; Dhalluin, A.; Cattoir, V.; Giard, J.-C. Landscape of resistance-nodulation-cell division (RND)-type efflux pumps in Enterobacter cloacae complex. Antimicrob. Agents Chemother. 2016. [Google Scholar] [CrossRef] [Green Version]
- Hernando-Amado, S.; Blanco, P.; Alcalde-Rico, M.; Corona, F.; Reales-Calderón, J.A.; Sánchez, M.B.; Martínez, J.L. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist. Update 2016, 28, 13–27. [Google Scholar] [CrossRef]
- Blanco, P.; Hernando-Amado, S.; Reales-Calderon, J.A.; Corona, F.; Lira, F.; Alcalde-Rico, M.; Bernardini, A.; Sanchez, M.B.; Martinez, J.L. Bacterial multidrug efflux pumps: Much more than antibiotic resistance determinants. Microorganisms 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housseini, B.; Issa, K.; Phan, G.; Broutin, I. Functional mechanism of the efflux pumps transcription regulators from Pseudomonas aeruginosa based on 3D structures. Front. Mol. Biosci. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olliver, A.; Vallé, M.; Chaslus-Dancla, E.; Cloeckaert, A. Role of an acrR mutation in multidrug resistance of in vitro-selected fluoroquinolone-resistant mutants of Salmonella enterica serovar Typhimurium. FEMS Microbiol. Lett. 2004, 238, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, M.A.; Talukder, A.; Piddock, L.J.V. Contribution of mutation at amino acid 45 of AcrR to acrB expression and ciprofloxacin resistance in clinical and veterinary Escherichia coli isolates. Antimicrob. Agents Chemother. 2005, 49, 4390–4392. [Google Scholar] [CrossRef] [Green Version]
- Ferrand, A.; Vergalli, J.; Pagès, J.-M.; Davin-Regli, A. An intertwined network of regulation controls membrane permeability Including drug influx and efflux in Enterobacteriaceae. Microorganisms 2020, 8, 833. [Google Scholar] [CrossRef]
- Ziha-Zarifi, I.; Llanes, C.; Köhler, T.; Pechere, J.-C.; Plesiat, P. In vivo emergence of multidrug-resistant mutants of Pseudomonas aeruginosa overexpressing the active efflux system MexA-MexB-OprM. Antimicrob. Agents Chemother. 1999, 43, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.P.; Hächler, H.; Levy, S.B. Genetic and functional analysis of the multiple antibiotic resistance (mar) locus in Escherichia coli. J. Bacteriol. 1993, 175, 1484–1492. [Google Scholar] [CrossRef] [Green Version]
- Wand, M.E.; Jamshidi, S.; Bock, L.J.; Rahman, K.M.; Sutton, J.M. SmvA is an important efflux pump for cationic biocides in Klebsiella pneumoniae and other Enterobacteriaceae. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Guérin, F.; Gravey, F.; Plésiat, P.; Aubourg, M.; Beyrouthy, R.; Bonnet, R.; Cattoir, V.; Giard, J.-C. The transcriptional repressor smvr is important for decreased chlorhexidine susceptibility in Enterobacter cloacae complex. Antimicrob. Agents Chemother. 2019, 64, e01845-19, /aac/64/1/AAC.01845-19.atom. [Google Scholar] [CrossRef]
- Pelling, H.; Bock, L.J.; Nzakizwanayo, J.; Wand, M.E.; Denham, E.L.; MacFarlane, W.M.; Sutton, J.M.; Jones, B.V. Derepression of the smvA efflux system arises in clinical isolates of Proteus mirabilis and reduces susceptibility to chlorhexidine and other biocides. Antimicrob. Agents Chemother. 2019, 63, e01535-19. [Google Scholar] [CrossRef] [Green Version]
- Villagra, N.A.; Hidalgo, A.A.; Santiviago, C.A.; Saavedra, C.P.; Mora, G.C. SmvA, and not AcrB, is the major efflux pump for acriflavine and related compounds in Salmonella enterica serovar Typhimurium. J. Antimicrob. Chemother. 2008, 62, 1273–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laborda, P.; Alcalde-Rico, M.; Blanco, P.; Martínez, J.L.; Hernando-Amado, S. Novel inducers of the expression of multidrug efflux pumps that trigger Pseudomonas aeruginosa transient antibiotic resistance. Antimicrob. Agents Chemother. 2019, 63, e01095-19. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Sutton, J.M.; Rahman, K.M. Effectiveness of efflux pump inhibitors as biofilm disruptors and resistance breakers in Gram-negative (ESKAPEE) bacteria. Antibiotics 2019, 8, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renau, T.E.; Léger, R.; Flamme, E.M.; Sangalang, J.; She, M.W.; Yen, R.; Gannon, C.L.; Griffith, D.; Chamberland, S.; Lomovskaya, O.; et al. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 1999, 42, 4928–4931. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Watkins, W.J.; Landaverry, Y.; Léger, R.; Litman, R.; Renau, T.E.; Williams, N.; Yen, R.; Zhang, J.Z.; Chamberland, S.; Madsen, D.; et al. The relationship between physicochemical properties, in vitro activity and pharmacokinetic profiles of analogues of diamine-containing efflux pump inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 4241–4244. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Amoako, D.G. Carbonyl cyanide m-chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant Enterobacteriaceae. Front. Microbiol. 2017, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, T.; Sharma, A.; Akhter, J.; Pathania, R. The small molecule IITR08027 restores the antibacterial activity of fluoroquinolones against multidrug-resistant Acinetobacter baumannii by efflux inhibition. Int. J. Antimicrob. Agents 2017, 50, 219–226. [Google Scholar] [CrossRef]
- Opperman, T.J.; Kwasny, S.M.; Kim, H.-S.; Nguyen, S.T.; Houseweart, C.; D’Souza, S.; Walker, G.C.; Peet, N.P.; Nikaido, H.; Bowlin, T.L. Characterization of a novel pyranopyridine inhibitor of the AcrAB efflux pump of Escherichia coli. Antimicrob. Agents Chemother. 2014, 58, 722–733. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.T.; Kwasny, S.M.; Ding, X.; Cardinale, S.C.; McCarthy, C.T.; Kim, H.-S.; Nikaido, H.; Peet, N.P.; Williams, J.D.; Bowlin, T.L.; et al. Structure–Activity relationships of a novel pyranopyridine series of Gram-negative bacterial efflux pump inhibitors. Bioorg Med. Chem 2015, 23, 2024–2034. [Google Scholar] [CrossRef] [Green Version]
- Sjuts, H.; Vargiu, A.V.; Kwasny, S.M.; Nguyen, S.T.; Kim, H.-S.; Ding, X.; Ornik, A.R.; Ruggerone, P.; Bowlin, T.L.; Nikaido, H.; et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Prot. Nat. Acad. Sci. USA 2016, 113, 3509–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolau, D.P. Carbapenems: A potent class of antibiotics. Expert Opin. Pharm. 2008, 9, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.; Hojabri, Z.; Mahdian, R.; Nahaei, M.R.; Rahmati, M.; Hojabri, T.; Pirzadeh, T.; Pajand, O. Role of efflux pumps: MexAB-OprM and MexXY(-OprA), AmpC cephalosporinase and OprD porin in non-metallo-β-lactamase producing Pseudomonas aeruginosa isolated from cystic fibrosis and burn patients. Infect. Genet. Evol. 2014, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sobel, M.L.; Neshat, S.; Poole, K. Mutations in PA2491 (mexS) promote MexT-dependent mexEF-oprN expression and multidrug resistance in a clinical strain of Pseudomonas aeruginosa. J. Bacteriol. Res. 2005, 187, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breidenstein, E.B.M.; de la Fuente-Núñez, C.; Hancock, R.E.W. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011, 19, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Coyne, S.; Courvalin, P.; Périchon, B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob. Agents Chemother. 2011, 55, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Saxena, P.; Joshi, Y.; Rawat, K.; Bisht, R. Biofilms: Architecture, resistance, quorum sensing and control mechanisms. Indian J. Microbiol. 2019, 59, 3–12. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belanger, C.R.; Mansour, S.C.; Pletzer, D.; Hancock, R.E.W. Alternative strategies for the study and treatment of clinical bacterial biofilms. Emerg. Top. Life Sci. 2017, 1, 41–53. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Shahrour, H.; Pitts, B.; Stewart, P.S.; Sánchez-Gómez, S.; Martínez-de-Tejada, G. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci. Rep. 2019, 9, 3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, G.D. Antibiotic adjuvants: Rescuing antibiotics from resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef]
- Cox, G.; Koteva, K.; Wright, G.D. An unusual class of anthracyclines potentiate Gram-positive antibiotics in intrinsically resistant Gram-negative bacteria. J. Antimicrob. Chemother. 2014, 69, 1844–1855. [Google Scholar] [CrossRef] [Green Version]
- Corbett, D.; Wise, A.; Langley, T.; Skinner, K.; Trimby, E.; Birchall, S.; Dorali, A.; Sandiford, S.; Williams, J.; Warn, P.; et al. Potentiation of antibiotic activity by a novel cationic peptide: Potency and spectrum of activity of SPR741. Antimicrob. Agents Chemother. 2017, 61, e00200-17. [Google Scholar] [CrossRef] [Green Version]
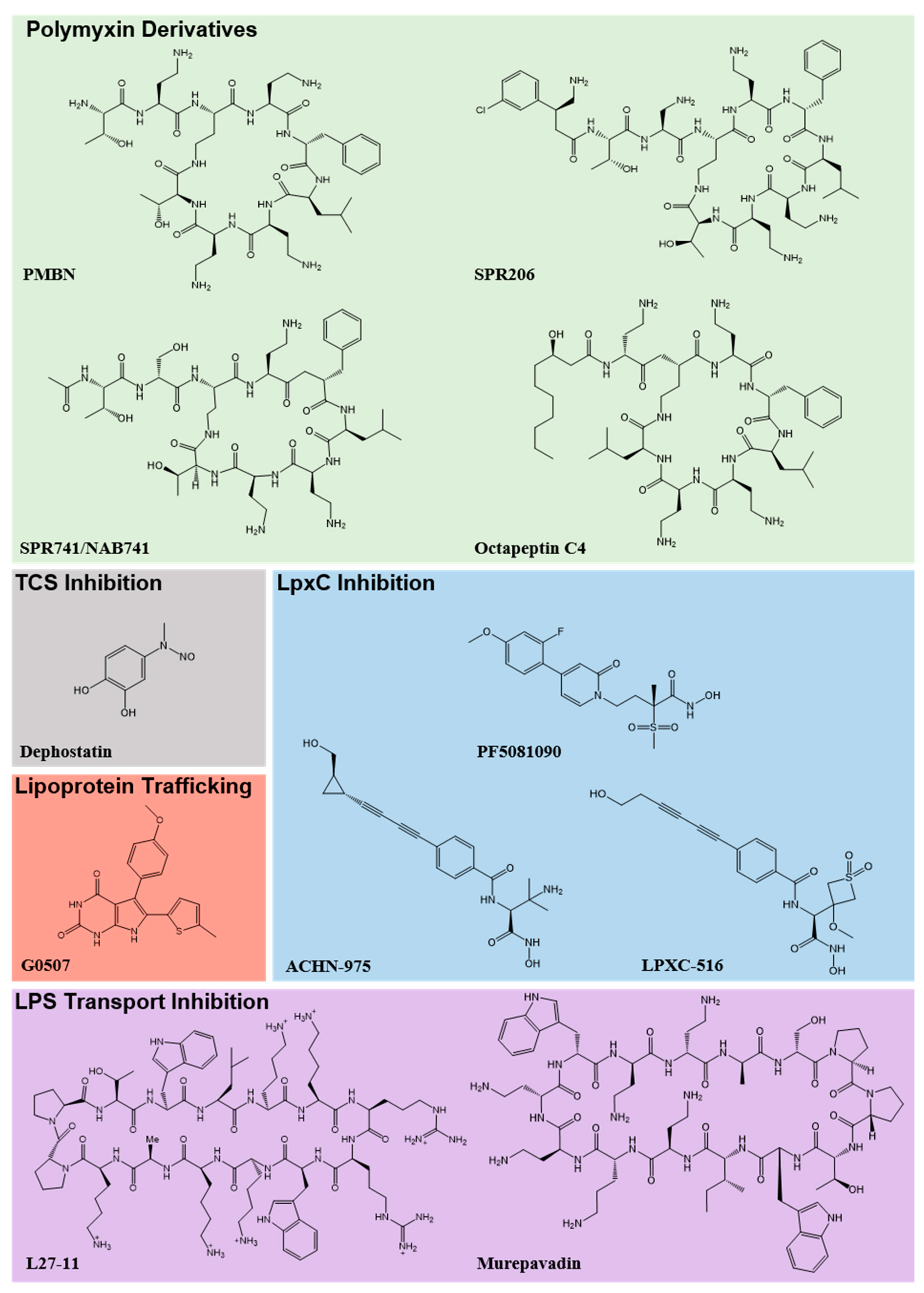

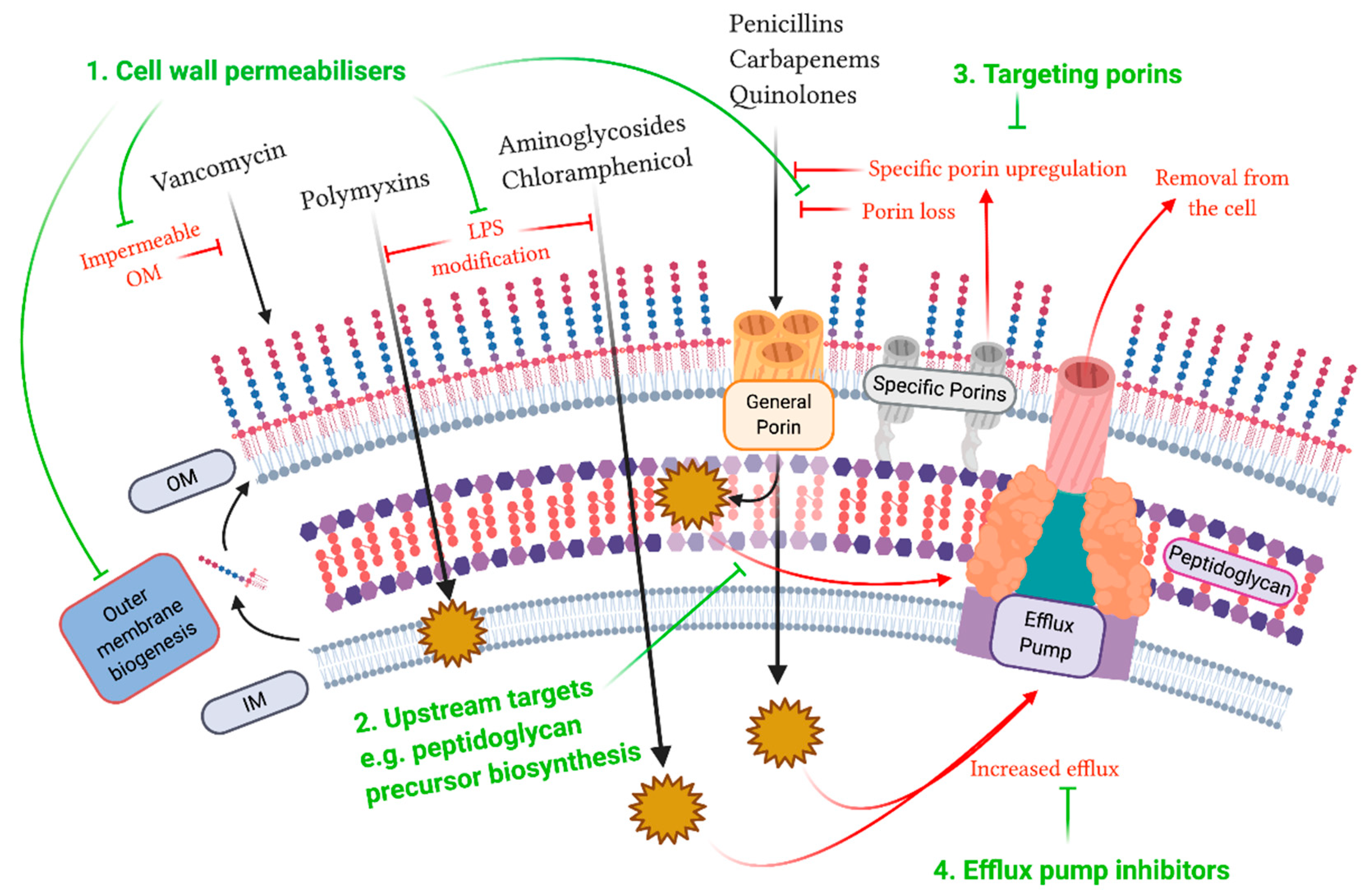
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Impey, R.E.; Hawkins, D.A.; Sutton, J.M.; Soares da Costa, T.P. Overcoming Intrinsic and Acquired Resistance Mechanisms Associated with the Cell Wall of Gram-Negative Bacteria. Antibiotics 2020, 9, 623. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090623
Impey RE, Hawkins DA, Sutton JM, Soares da Costa TP. Overcoming Intrinsic and Acquired Resistance Mechanisms Associated with the Cell Wall of Gram-Negative Bacteria. Antibiotics. 2020; 9(9):623. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090623
Chicago/Turabian StyleImpey, Rachael E., Daniel A. Hawkins, J. Mark Sutton, and Tatiana P. Soares da Costa. 2020. "Overcoming Intrinsic and Acquired Resistance Mechanisms Associated with the Cell Wall of Gram-Negative Bacteria" Antibiotics 9, no. 9: 623. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9090623




