Non-Invasive On-Site Raman Study of Pigments and Glassy Matrix of 17th–18th Century Painted Enamelled Chinese Metal Wares: Comparison with French Enamelling Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results
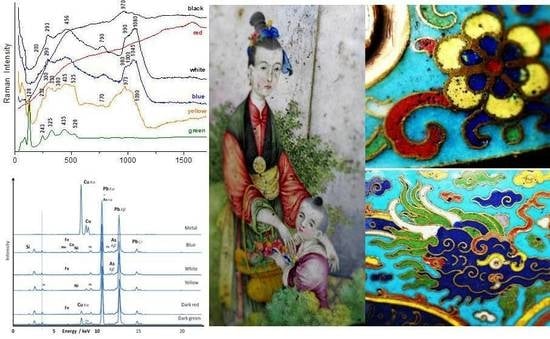
3.1. Cloisonné Enamels
3.1.1. Opacifiers
3.1.2. Pigments
- (i)
- a first type characterised with a low-wavenumber strongest peak at ~128–129 cm−1 and peaks of lower intensity at ~245, ~325, 435 and ~525 cm−1 (see e.g., Figure 5D right);
- (ii)
- a second type with the strongest peak at ~135–140 cm−1 and peaks of lower intensity at ~320–335, ~440–450 and ~520 cm−1 (see e.g., Figure 7A,C);
- (iii)
- a third one displaying similar features but with additional low intensity peaks at ~375, ~470 cm−1 (Figure 5B left);
- (iv)
- a fourth one displaying similar features with the others but with an additional strong component at ~510 cm−1 with its intensity being similar with that of the low wavenumber one at ~135 cm−1 (Figure 7A,C). This strong low wavenumber peak arises from the vibration of Pb2+ ions and its wavenumber position both depends on the composition and firing temperature [36,37,38,53,54,55,56,57,58,59,60,61,62]: the peak wavenumber decreases when the firing temperature increases.
3.1.3. Glassy Matrix
3.2. Painted Enamels
3.2.1. Opacifiers
3.2.2. Pigments
3.2.3. Glassy Matrix
3.2.4. Glass Coloured with Gold Metal Nanoparticles
3.3. Elemental Portable X-Ray Fluorescence Study
4. Discussion
- (i)
- cassiterite (SnO2), a very powerful white opacifier;
- (ii)
- blue glass coloured (partially or totally) with arsenic-rich European cobalt and voluntary use of arsenic as a promoter of opacification, particularly with precipitation of lead arsenate apatite [Na1-x-y/2KxCayPb4(AsO4)3];
- (iii)
- colloidal gold (Au° nanoparticles), called Cassius’ purple for purple to pink colour of European faience and porcelain enamels and ruby for glass, and characteristic of Qianlong Famille rose porcelains;
- (iv)
- the different pyrochlores (A2-xA’xB2-yB’yO7-δ), usually called Naples yellow, namely the antimony-rich and tin-rich phases and the quaternary complex phase with variable content of zinc, silicon and iron.
4.1. Cassiterite Opacification
4.2. Cobalt Ores’ Provenance
4.3. Gold Nanoparticles
4.4. Naples Yellow Lead Pyrochlore
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colomban, P. Glazes and Enamels. In Encyclopedia of Glass Science, Technology, History, and Culture; Richet, P., Ed.; J. Wiley & Sons Inc.: New York, NY, USA, 2020; Chapter 10.6. [Google Scholar]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Gauthier, M.-M. Émaux. In Encyclopédie Universalis; Encyclopédie Universalis: Paris, France, 1985; Volume 6, pp. 939–960. [Google Scholar]
- Cooper, E. Ten Thousand Years of Pottery, 4th ed.; University of Pennsylvania Press: Philadelphia, PA, USA, 2000. [Google Scholar]
- Moorey, P.R.S. Ancient Mesopotamian Materials and Industries, The Archaeological Evidence; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Roehrig, C.H. Glass. In Hatshepsut: From Queen to Pharaoh; Roehrig, C.H., Dreyfus, R., Keller, C.A., Eds.; The Metropolitan Museum of Art: New York, NY, USA, 2005; pp. 67–69. [Google Scholar]
- Pierides, A. Jewellery in the Cyprus Museum; Republic of Cyprus the Department of Antiquities: Cyprus, Cyprus, 1971. [Google Scholar]
- Ward, G.W.R. Enamel. In The Grove Encyclopedia of Materials and Techniques in Art; Oxford University Press: Oxford, UK, 2008; pp. 187–190. [Google Scholar]
- Campbell, M. An Introduction to Medieval Enamels; HMSO: London, UK, 1983. [Google Scholar]
- Kruta, V. The first Celtic expansion: Prehistory to history. In The Celts; Moscati, S., Ed.; Bompiani: Milano, Italy, 1991; pp. 195–210. [Google Scholar]
- Buckton, D. Byzantine Enamel. In Byzantium: Treasures of Byzantine Art and Culture; Buckton, D., Ed.; The British Museum Press: London, UK, 1994; p. 18. [Google Scholar]
- Kırmızı, B.; Colomban, P.; Quette, B. On-site analysis of Chinese Cloisonné enamels from fifteenth to nineteenth centuries. J. Raman Spectrosc. 2010, 41, 780–790. [Google Scholar]
- Colomban, P.; Arberet, L.; Kırmızı, B. On-site Raman analysis of 17th and 18th century Limoges enamels: Implications on the European cobalt sources and the technological relationship between Limoges and Chinese enamels. Ceram. Int. 2017, 43, 10158–10165. [Google Scholar] [CrossRef] [Green Version]
- Quette, B. (Ed.) Cloisonné: Chinese Enamels from the Yuan, Ming and Qing Dynasties; Bard Catalogue, Yale University Press: Yale, CT, USA, 2011. [Google Scholar]
- Garner, S.H.M. Chinese and Japanese Cloisonné Enamels; Faber & Faber: London, UK, 1962. [Google Scholar]
- Zhou, L.L. Discussion on falangcai Enamels—And the Difference between falangcai and yangcai. Shanghai Bowuguan Jikan 2000, 8, 210–226. [Google Scholar]
- Shih, C.F. Evidence of East-West exchange in the eighteenth century: The establishment of painted enamel art at the Qing Court in the reign of Emperor Kangxi. Natl. Palace Mus. Res. Q. 2007, 24, 45–94. [Google Scholar]
- Zhou, S.Z. Research on Painted Enamels Porcelain Ware from the Qing Court; Wenwu Chubanshe: Beijing, China, 2008. [Google Scholar]
- Lili, F. La Céramique Chinoise; China Intercontinental Press: Beijing, China, 2011. [Google Scholar]
- Shih, C.F. Radiant Luminance: The Painted Enamelware of the Qing Imperial Court; The National Palace Museum of Taipei: Taipei, Taiwan, 2012. [Google Scholar]
- Xu, X.D. Europe-China-Europe: The Transmission of the Craft of Painted Enamel in the Seventeenth and Eighteenth Centuries. In Goods from the East, 1600–1800 Trading Eurasia; Berg, M., Gottmann, F., Hodacs, H., Nierstrasz, C., Eds.; Palgrave Macmillan: London, UK, 2015; pp. 92–106. [Google Scholar]
- Zhao, B.; Wang, G.Y.; Biron, I.; Colomban, P.; Hilaire-Pérez, L. La circulation des techniques de l′émail entre la France et la Chine du XVIIème au XIXème siècle. Le CNRS en Chine Bull. 2016, 21, 20–25. Available online: http://www.cnrs.fr/derci/IMG/pdf/cnrsenchine_21_fr_final_pour_le_site_cnrs.pdf (accessed on 15 December 2019).
- Curtis, E.B. A plan to the Emperor’s glassworks. Arts Asiatiques 2001, 56, 81–90. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020. [Google Scholar] [CrossRef]
- Blanc, M.; Biron, I.; Colomban, P.; Notin, V. Emaux Peints de Limoges, XVe-XVIIIe siècles—La Collection du Musée des Arts Décoratifs; Les Arts Décoratifs: Paris, France, 2011. [Google Scholar]
- Kırmızı, B.; Colomban, P.; Blanc, M. On-site analysis of Limoges enamels from sixteenth to nineteenth centuries: An attempt to differentiate between genuine artefacts and copies. J. Raman Spectrosc. 2010, 41, 1240–1247. [Google Scholar] [CrossRef]
- Colomban, P.; Zhang, Y.; Zhao, B. Non-invasive Raman analyses of huafalang and related porcelain wares. Searching for evidence for innovative pigment technologies. Ceram. Int. 2017, 43, 12079–12088. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ambrosi, F.; Ngo, A.-T.; Lu, T.-A.; Feng, X.-L.; Chen, S.; Choi, C.-L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram. Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Kırmızı, B. Non-invasive on-site Raman study of polychrome and white enamelled glass artefacts in imitation of porcelain assigned to Bernard Perrot and his followers. J. Raman Spectrosc. 2020, 51, 133–146. [Google Scholar] [CrossRef]
- Colomban, P.; Lu, T.-A.; Milande, V. Non-invasive on-site Raman study of blue-decorated early soft-paste porcelain: The use of arsenic-rich (European) cobalt ores—Comparison with huafalang Chinese porcelains. Ceram. Int. 2018, 44, 9018–9026. [Google Scholar] [CrossRef]
- Caggiani, M.-C.; Colomban, P. Testing of Raman spectroscopy as a non-invasive tool for the investigation of glass-protected pastels. J. Raman Spectrosc. 2011, 42, 790–798. [Google Scholar] [CrossRef]
- Mancini, D.; Tournié, A.; Caggiani, M.-C.; Colomban, P. Testing of Raman spectroscopy as a non-invasive tool for the investigation of glass-protected miniature portraits. J. Raman Spectrosc. 2012, 43, 294–302. [Google Scholar] [CrossRef]
- Henderson, J.; Tregear, M.; Wood, N. The technology of sixteenth- and seventeenth century Chinese cloisonné enamels. Archaeometry 1989, 31, 133–146. [Google Scholar] [CrossRef]
- Su, Y.; Qu, L.; Duan, H.; Tarcea, N.; Shen, A.; Popp, J.; Hu, J. Elemental analysis-aided Raman spectroscopic studies on Chinese cloisonné wares and Painted enamels from the Imperial Palace. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 153, 165–170. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B. Investigation of the pigments and glassy matrix of the 18th century painted enamelled Chinese porcelains by non-invasive on-site Raman microspectometry. 2020. Unpublished work. [Google Scholar]
- Montanari, R.; Alberghina, M.F.; Casanova Municchia, A.; Massa, E.; Pelagotti, A.; Pelosi, C.; Schiavone, S.; Sodo, A. A polychrome Mukozuke (1624–1644) porcelain offers a new hypothesis on the introduction of European enameling technology in Japan. J. Cult. Herit. 2017, 32, 232–237. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. The Origin of overglaze-blue enameling in Japan: New discoveries and a reassessment. J. Cult. Herit. 2019, 37, 94–102. [Google Scholar] [CrossRef]
- Montanari, R.; Murakami, N.; Colomban, P.; Alberghina, M.F.; Pelosi, C.; Schiavone, S. European Ceramic technology in the Far East: Enamels and pigments in Japanese art from the 16th to the 20th century and their reverse influence on China. Herit. Sci. 2020. Submitted. [Google Scholar]
- Wood, N. Chinese Glazes: Their Origins, Chemistry and Recreation; A & C Black: London, UK, 1999; pp. 194–195. [Google Scholar]
- Gan, F. Origin and Evolution of Ancient Chinese Glass. In Ancient Glass Research Along the Silk Road; Gan, F., Brill, R., Shouyun, T., Eds.; World Scientific Publishing Co: Singapore, 2009. [Google Scholar]
- Cui, J.; Wu, X.; Huang, B. Chemical and lead isotope analysis of some lead-barium glass wares from the Warring States Period, unearthed from Chu tombs in Changde City, Hunan Province, China. J. Archaeol. Sci. 2011, 38, 1671–1679. [Google Scholar] [CrossRef]
- Kingery, W.D.; Vandiver, P.B. The Eighteenth-Century Change in Technology and Style from the Famille-Verte Palette to the Famille-Rose Palette. In Technology and Style; Kingery, W.D., Ed.; Ceramics and Civilization Series; The American Ceramic Society: Colombus, OH, USA, 1986; Volume 2, pp. 363–381. [Google Scholar]
- Van Pevenage, J.; Lauwers, D.; Herremans, D.; Verhaeven, E.; Vekemans, B.; De Clercq, W.; Vincze, L.; Moens, L.; Vandenabeele, P. A Combined Spectroscopic Study on Chinese Porcelain Containing Ruan-Cai Colours. Anal. Methods 2014, 6, 387–394. [Google Scholar] [CrossRef]
- Giannini, R.; Freestone, I.C.; Shortland, A.J. European cobalt sources identified in the production of Chinese famille rose porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, J.D.; McCreery, R.L. In situ Raman microscopy of chromate effects on corrosion pits in aluminum alloy. J. Electrochem. Soc. 1999, 146, 4076–4081. [Google Scholar] [CrossRef]
- Frost, R.L. Raman Microscopy of Selected Chromate Minerals. J. Raman Spectrosc. 2004, 35, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Milande, V.; Le Bihan, L. On-site Raman Analysis of Iznik pottery glazes and pigments. J. Raman Spectrosc. 2004, 35, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Koleini, F.; Colomban, P.; Pikirayi, I.; Prinsloo, L.C. Glass beads, markers of ancient trade in sub-Saharan Africa: Methodology, State of the Art and Perspectives. Heritage 2019, 2, 144. [Google Scholar] [CrossRef] [Green Version]
- Ricciardi, P.; Colomban, P.; Tournié, A.; Macchiarola, M.; Ayed, N. A non-invasive study of Roman Age mosaic glass tesserae by means of Raman spectroscopy. J. Archaelog. Sci. 2009, 36, 2551–2559. [Google Scholar] [CrossRef]
- Neri, E.; Morvan, C.; Colomban, P.; Guerra, M.F.; Prigent, V. Late Roman and Byzantine mosaic opaque “glass-ceramics” tesserae (5th–9th century). Ceram. Int. 2016, 42, 18859–18869. [Google Scholar] [CrossRef] [Green Version]
- Kırmızı, B.; Göktürk, H.; Colomban, P. Colouring agents in the pottery glazes of western Anatolia: A new evidence for the use of Naples yellow pigment variations during the late Byzantine period. Archaeometry 2015, 57, 476–496. [Google Scholar] [CrossRef]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S. Lead-tin-antimony yellow-Historical manufacture, molecular characterization and identification in seventeenth-century Italian paintings. Stud. Conserv. 2004, 49, 41–52. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Ricciardi, P.; Colomban, P.; Tournié, A.; Milande, V. Non-destructive on-site identification of ancient glasses: Genuine artefacts, embellished pieces or forgeries? J. Raman Spectrosc. 2009, 40, 604–617. [Google Scholar] [CrossRef]
- Pereira, M.; de Lacerda-Aroso, T.; Gomes, M.J.M.; Mata, A.; Alves, L.C.; Colomban, P. Ancient Portuguese ceramic wall tiles («Azulejos»): Characterization of the glaze and ceramic pigments. J. Nano Res. 2009, 8, 79–88. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial yellow pigments: Production and characterization through spectroscopic methods of analysis. e-Preserv. Sci. 2010, 7, 108–115. [Google Scholar]
- Rosi, F.; Manuali, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamellotti, A.; Burgio, L.; Seccaroni, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar]
- Cartechini, L.; Rosi, F.; Miliani, C.; D’Acapito, F.; Brunetti, B.G.; Sgamellotti, A. Modified Naples yellow in Renaissance majolica: Study of Pb-Sb-Zn and Pb-Sb-Fe ternary pyroantimonates by X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2011, 26, 2500–2507. [Google Scholar] [CrossRef]
- Colomban, P.; Maggetti, M.; d’Albis, A. Non-invasive Raman identification of crystalline and glassy phases in a 1781 Sèvres Royal Factory soft paste porcelain plate. J. Eur. Ceram. Soc. 2018, 38, 5228–5233. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Louhichi, A.; Binous, H.; Ayed, N. Identification par Microscopie Raman des Tessons et Pigments de Glaçures de Céramiques de l’Ifriqiya (Dougga: XIe-XVIIIe siècles). Revue d’Archéomètrie 2001, 25, 101–112. [Google Scholar] [CrossRef]
- Jehlicka, J.; Vitek, P.; Edwards, H.G.M.; Hargreaves, M.D.; Capoun, T. Fast detection of sulphate minerals (gypsum, anglesite, baryte) by a portable Raman spectrometer. J. Raman Spectrosc. 2009, 40, 1082–1086. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewellery, ceramic enamels and mosaics. J. Non-Crystall. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Colomban, P.; Paulsen, O. Non-destructive Raman Determination of the Structure and Composition of Glazes by Raman Spectroscopy. J. Amer. Ceram. Soc. 2005, 88, 390–395. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramic, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. Non-Destructive Raman Analysis of Ancient Glasses and Glazes, in Modern Methods for Analysing Archaeological and Historical Glass, 1st ed.; Janssens, K., Ed.; John Wiley & Sons Ltd: London, UK, 2012; pp. 275–300. [Google Scholar]
- Labet, V.; Colomban, P. Vibrational properties of silicates: A cluster model able to reproduce the effect of “SiO4” polymerization on Raman intensities. J. Non-Crystall. Solids 2013, 370, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Manoun, B.; Azdouz, M.; Azrour, M.; Essehli, R.; Benmokhtar, S.; El Ammari, L.; Ezzahi, A.; Ider, A.; Lazor, P. Synthesis, Rietveld refinements and Raman spectroscopic studies of tricationic lacunar apatites Na1−xKxPb4(AsO4)3 (0 < x < 1). J. Mol. Struct. 2011, 986, 1–9. [Google Scholar]
- Bernardini, S.; Bellatreccia, F.; Municchia, A.C.; Della Ventura, G.; Sodo, A. Raman spectra of natural manganese oxides. J. Raman Spectrosc. 2019, 50, 873–888. [Google Scholar] [CrossRef]
- Geyssant, J. Secret du verre rouge transparent de Bernard Perrot et comparaison avec celui de Johann Kunckel. In Bernard Perrot (1640–1709), Secrets et Chefs-d’œuvre des Verreries Royales d’Orléans; Catalogue, Klinka Ballesteros, I., de Valence, C., Maitte, C., Ricke, H., Eds.; Musée des Beaux-Arts d’Orléans—SOMOGY Editions d’Arts: Paris, France, 2013; pp. 51–54. [Google Scholar]
- Colomban, P. The Use of Metal Nanoparticles to Produce Yellow, Red and Iridescent Colour, from Bronze Age to Present Times in Lustre Pottery and Glass: Solid State Chemistry, Spectroscopy and Nanostructure. J. Nano Res. 2009, 8, 109–132. [Google Scholar] [CrossRef] [Green Version]
- Hunt, L.B. The true story of Purple of Cassius. Gold Bull. 1976, 9, 134–139. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W. Glass and Enamel by Preparations of Gold, Commercium Philosophico Technicum or The Philosophical Commerce of Arts: Designed as an Attempt to Improve Arts, Trades, and Manufactures; London, UK, 1763; p. 170. [Google Scholar]
- Jiang, X.; Ma, Y.; Chen, Y.; Li, Y.; Ma, Q.; Zhang, Z.; Wang, C.; Yang, Y. Raman analysis of cobalt blue pigment in blue and white porcelain: A reassessment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Schreiber, H. Raman Signature Modification Induced by Copper Nanoparticles in Silicate Glass. J. Raman Spectrosc. 2005, 36, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Tournié, A.; Ricciardi, P. Raman spectroscopy of copper nanoparticle-containing glass matrices: The ancient red stained-glass windows. J. Raman Spectrosc. 2009, 40, 1949–1955. [Google Scholar] [CrossRef]
- Sciau, P.; Noé, L.; Colomban, P. Metal nanoparticles in contemporary potters’ master pieces: Lustre and red “pigeon blood” potteries: Models to understand the ancient technology. Ceram. Int. 2016, 42, 15349–15357. [Google Scholar] [CrossRef]
- Brun, N.; Mazerolles, L.; Pernot, M. Microstructure of opaque red glass containing copper. J. Mater. Sci. Lett. 1991, 10, 1418–1420. [Google Scholar] [CrossRef]
- Kingery, W.D.; Vandiver, P.B. Song Dynasty Jun (Chun) ware glazes. Am. Ceram. Bull. 1983, 62, 1269–1274. [Google Scholar]
- Freestone, I.C.; Barber, D.J. The Development of the Colour of Sacrificial Red Glaze with Special Reference to a Qing Dynasty Saucer Dish. In Chinese Copper Red Wares; Scott, R.E., Ed.; Percival David Foundation of Chinese Art, Monograph Series N°3; University of London, School of Oriental and African Art: London, UK, 1992; pp. 53–62. [Google Scholar]
- Yang, Y.M.; Feng, M.; Ling, X.; Mao, Z.Q.; Wang, C.S.; Sun, X.M.; Guo, M. Microstructural analysis of the color-generating mechanism in Ru ware, modern copies and its differentiation with Jun ware. J. Archaeol. Sci. 2005, 32, 301–310. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yang, Y.M.; Zhu, J.; Zhang, X.G.; Jiang, S.; Zhang, Z.X.; Yao, Z.Q.; Solbrekken, G. Colour-generating mechanism of copper-red porcelain from Changsha Kiln (AD 7th–10th century), China. Ceram. Int. 2016, 42, 8495–8500. [Google Scholar] [CrossRef]
- Wayne Richardson, H.W. Handbook of Copper Compounds and Applications; CRC Press: Boca Raton, FL, USA, 1997; pp. 154–155. [Google Scholar]
- Colomban, P.; Treppoz, F. Identification and Differentiation of Ancient and Modern European Porcelains by Raman Macro- and Microspectroscopy. J. Raman Spectrosc. 2001, 32, 93–102. [Google Scholar] [CrossRef]
- Colomban, P.; Robert, I.; Roche, C.; Sagon, G.; Milande, G. Identification des porcelaines « tendres » du 18e siècle par spectroscopie Raman: Saint-Cloud, Chantilly, Mennecy et Vincennes/Sèvres. Rev. d’Archéomtrie 2004, 28, 153–167. [Google Scholar] [CrossRef]
- Magetti, M.; d’Albis, A. Phase and compositional analysis of a Sèvres sof paste porcelain plate from 1781, with a review of early porcelain techniques. Eur. J. Mineral. 2017, 29, 347–367. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P. Rocks as blue, green and black pigments/dyes of glazed pottery and enamelled glass artefacts –A review. Eur. J. Mineral. 2014, 25, 863–879. [Google Scholar] [CrossRef]
- Kissin, S.A. Five element (Ni-Co-As-Ag-Bi) veins. Geosci. Can. 1992, 19, 113–124. Available online: https://journals.lib.unb.ca/index.php/gc/article/view/3768/4282/ (accessed on 15 December 2019).
- Mancini, D.; Dupont-Logié, C.; Colomban, P. On-site identification of Sceaux porcelain and faience using a portable Raman instrument. Ceram. Int. 2016, 42, 14918–14927. [Google Scholar] [CrossRef] [Green Version]
- Merrifield, M.P. Medieval and Renaissance Treatises on the Arts of Painting (1849); Reprint: New York NY, USA, 1967; p. 334. [Google Scholar]
- Bertran, H.; Reboulleau; Magnier; Romain, A. Nouveau Manuel Complet de la Peinture sur Verre, sur Porcelaine et sur émail; Encyclopédie-Roret, L. Mulo: Paris, France, 1913. [Google Scholar]
- Brill, R.H.; Cahill, N.D. A red opaque glass from Sardis and some thoughts on red opaques in general. J. Glass Stud. 1988, 30, 16–27. [Google Scholar]
- Maltoni, S.; Silvestri, A. A Mosaic of Colors. Investigating Production Technologies of Roman Glass Tesserae From Northeastern Italy. Minerals 2018, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; March, G.; Mazerolles, L.; Karmous, T.; Ayed, N.; Ennabli, A.; Slim, H. Raman identification of materials used for jewellery and mosaics in Ifriqiya. J. Raman Spectrosc. 2003, 34, 205–213. [Google Scholar] [CrossRef]
- Freestone, I.C.; Meek, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman nanotechnology. Gold Bull. 2007, 40, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Epler, R.A.; Epler, D.R. Glazes and Glass Coatings; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Shi, N.C. Analyse scientifique des couleurs falang d’objets conservés au Palais ou retrouvés en contexte archéologique. In Proceedings of the French-Chinese Meeting on Enamelling Technology, Paris, France, 10 September 2019. [Google Scholar]















| Artefacts | Inventory Number | Collection/ Provenance | Place of Production (Expected) | Period | Size (cm) | Images |
|---|---|---|---|---|---|---|
| Incense tripod, cloisonné enamel | F1448C | Fontainebleau (Musée chinois) | ? | Kangxi period (1662–1722) | H:27 D:26 |  |
| High vase, cloisonné enamel | F1735C | Palace workshop | Qianlong mark period (1736–1795) | H:103 D:40 |  | |
| Bottle, cloisonné enamel | F1501 | Mark?3rd quarter of 18th century | H: 21 D: 15 |  | ||
| Ewer, painted and cloisonné enamel on gold body | F1467.1 | 3rd quarter of 18th century Qianlong mark | H: 40 D: 20 A: 27 |  | ||
| Ewer, with painted and cloisonné enamel on gold body | F1467.2 | 3rd quarter of 18th century Qianlong mark |  | |||
| Vase, painted enamel on porcelain | F1341C | Jingdezhen Imperial Factory of Porcelain | mid-2nd half of 18th century End of Yongzheng period | H: 30 D: 15 |  | |
| Dish, painted enamel on copper body | R957 | Louvre (Département des Objets d’Art) | Guangzhou | (1723–1735)—early Qianlong period: mid-18th century | H: 3 D: 15.5 |  |
| Dish, painted enamel on copper body | R958 | Guangzhou | Qianlong period: 2nd half of 18th century | H: 2 D: 19 |  | |
| Bottle, painted enamel on copper body (pXRF studied) | R975 | Guangzhou | Qianlong period: 2nd half of 18th century | H: 26.5 D: 14 |  | |
| Incense tripod, painted enamel on copper body | F1698C | Fontainebleau (Musée chinois) | Guangzhou | Qianlong period (1736–1795): 2nd half of 18th century | H: 12.5 D: 14 |  |
| Bottle, painted enamel | F1440C | - | H: 21.5 D: 10 |  | ||
| Tea pot, of so- called ‘Thousand flowers Pattern’ | F1429C | Jingdezhen Imperial Factory of Porcelain | H: 13 D: 12 A: 19 |  |
| Phase | Characteristic Raman Peaks (cm−1) | Refs. | Artefact | Colour | Remarks |
|---|---|---|---|---|---|
| Naples Yellow lead pyrochlore | 95(?), 135, 251, 337, 375, 470 | [24,58,59,60,61,62] | F1448C | L.green | Complex Sb–Sn |
| 135, 320, 435 | F1448C | Yellow | Sn-rich | ||
| 128, 243, 325, 445, 525 | F1501 | Green | Sn-rich | ||
| 128, 246, 325, 380, 435, 525 | F1501 | Yellow | Complex Sb–Sn | ||
| 129,245,325,435,530 | F1735C | Yellow | Sn-rich | ||
| 129, 245, 325, 430, 475, 530 | F1735C | Green | Complex Sb–Sn | ||
| 136, 253, 330, 445, 520 | F1467.1 | Yellow | Sn-rich | ||
| 130, 340, 385, 455, 509 | F1467.1 | Yellow g | Complex Sb-rich | ||
| 135, 255, 330, 450, 520 | F1467.1 | Green | Sn-rich | ||
| 140, 255, 335, 380, 470, 525 | F1467.1 | Yellow-or | Complex Sb–Sn | ||
| 134, 250, 328, 445 | F1467.1 | Yellow-or | Sn-rich | ||
| Cassiterite | 631, 780 | [2] | F1448C | Yellow, green yellow (l green) | - |
| Lead arsenate | 825 | [13,27,28,44] | F1501 | Blue | As-rich cobalt |
| apatite | 815 | F1467.1 | Dark pink | Colloidal Au° | |
| Phosgenite | 1045 | [63] | F1735C | White Red | Corrosion product/restoration? |
| Chromate | 315, 403, 875 | [45,46] | F1448C | Blue | - |
| Artefact | Si–O Stretching Components (cm−1) | Colour | Glass Type |
|---|---|---|---|
| F1448C | 900, 975, 1040, 1150 | White | Lead-alkali IIb |
| 920, 990, 1065 | White pink | Lead-alkali III | |
| 990, 1100, 1150 | Blue | Lead-alkali IV | |
| 980, 1070, 1110 | L. green yellow | Lead-alkali III | |
| 1000, 1093 | Green | Lead-alkali IV | |
| F1735C | 985, 1045, 1095 | White, blue | Lead-alkali IIa |
| 980, 1030, 1100 | Green | - | |
| 980,1050, 1100 | Violet | - | |
| F1501 | 990, 1080 | White | Lead-alkali IV |
| 980, 1045, 1100 | Blue | Lead-alkali IIa | |
| 900, 975, 1040, 1090 | Yellow | Lead-rich I | |
| 970, 1040 | Green | Lead-alkali IIb | |
| 970, 1030, 1100 | Black | Lead-rich I | |
| F1467.1 | 980, 1065 | Turquoise | Lead-alkali III |
| 910, 985, 1070, 1150 | Green | - | |
| 975, 1070, 1145 | Blue | - |
| Phase | Characteristic Raman Peak (cm−1) | Refs. | Artefact | Colour |
|---|---|---|---|---|
| Lead arsenate apatite | 775, 813–815 | [13,27,28,43,62] | F1341C | White, blue, green, yellow, red |
| 765,810 | F1429C | White, blue, pink, yellow, green, black | ||
| 810 | F1698C | White, light blue, green, dark green | ||
| 820 | F1440C | Black, blue, white, light green, green, yellow, orange, red | ||
| 810 | F1440C | Dark green | ||
| 775,810 | R975 | White, blue, pink, yellow, green | ||
| 775, 815 | R957 | White, blue, yellow, green | ||
| 805 | R957 | Dark blue | ||
| 775, 815 | R958 | White, blue, yellow, green, red | ||
| 775-790,820–825 | F1467.1 | White, blue, yellow, green | ||
| Manganese oxides | 340, 550, 580, 892, 950 | [48,71] | F1429C | Black, brown |
| Naples Yellow lead pyrochlore | 126, 340, 445 | [12,24,58,59,60,61,62] | F1341C | Light green, yellow |
| 133, 340, 460 | F1341C | Yellow dot | ||
| 134, 330, 450, 490, 518 | F1429C | Yellow | ||
| 134, 260, 325, 440 | F1698C | Green, green yellow | ||
| 123, 345, 510 | F1440C | Yellow, dark green | ||
| 137, 252, 335 | F1440C | Yellow (frame) | ||
| 133, 328, 436, 510 | R975 | Yellow, green | ||
| 133, 315, 440 | R957 | Yellow, green | ||
| 132, 325, 450 | R958 | Yellow, green | ||
| 136, 335, 450, 520 | F1467.1 | Green (S. Medallion) | ||
| 133, 343, 390, 452, 508 | F1467.1 | Green | ||
| 133, 340, 380, 455, 506 | F1467.1 | Yellow | ||
| Hematite | 212, 290, 408, 490, 605, 1310 | [2,48,49] | F1341C | Red-orange |
| 223, 243, 292, 410, 1315 | F1440C | Orange, yellow | ||
| 225, 410, 1318 | F1467.1 | Orange | ||
| 222, 410, 1312 | F1429C | Brown | ||
| 222, 290, 405, 602, 1305 | F1429C | Red | ||
| Quartz | 463 | [2] | F1429C | Dark yellow green |
| Copper oxide? | 300 | [2] | F1467.1 | Black |
| Cassiterite | 632, 775 | [2] | F1467.1 | Green (S. Medallion) |
| Artefact | Si–O Stretching Components (cm−1) | Colour | Glass Type |
|---|---|---|---|
| F1467.1 | 980, 1040, 1070, 1150 | Green (medallion) | Lead-alkali IIb |
| 975, 1040, 1130 | Green | “ | |
| 980, 1040 | Yellow,red | Lead-reach alkali IIb | |
| R957 | 978, 1034, 1130 | White,blue | Lead-alkali IIb |
| 920, 965, 1030, 1140 | Yellow | Lead-alkali IIb | |
| R958 | 970, 1030, 1130 | Yellow,green,red | Lead-alkali IIb |
| 915,985, 1025, 1135 | Green | Lead-alkali IIb | |
| R975 | 975, 1045, 1130 | White | Lead-alkali IIb |
| 920, 972, 1038, 1130 | Yellow,green | Lead-alkali IIb | |
| 1000 | Turquoise | Lead-rich Ib | |
| F1698C | 980, 1045, 1140 | White,blue,green | Lead-alkali IIb |
| F1440C | 980, 1070, 1150 | Yellow,green | Lead-alkali III |
| 980, 1040 | Yellow | Lead-alkali IIa | |
| F1429C (porcelain) | 980, 1030, 1140 | White,blue | Lead-alkali IIa |
| 970, 1020 | Light pink | Lead-alkali IIa | |
| 900, 975, 1010, 1130 | Yellow | Lead-rich Ib | |
| 900, 975, 1010, 1125 | Green | Lead-alkali IIb | |
| F1341C (porcelain) | 970, 1035 | White,blue | Lead-alkali IIb |
| 980, 1030 | Green | Lead-alkali IIb | |
| 1040, 1090 | Red | Lead-alkali IIa |
| Colour | Elements |
|---|---|
| Blue | Si, Pb—Co, Fe, As, Sn—Ni, Mn |
| White | Si, Pb—As, Sn—Fe,Ni |
| Yellow | Si, Pb—As, Cu, Sn—Sb, Fe, Ni |
| Dark red | Si, Pb—Cu, Sn, As—Fe, Ni |
| Dark green | Si, Pb—Cu, Sn, As—Fe, Co, Ni, Mn |
| Criteria | Main Raman Peak (cm−1) | Artefact | Colour | Europe | Refs. | China | Refs |
|---|---|---|---|---|---|---|---|
| Colloidal gold | Background peaking at ~500–600 nm | Glass | Pink, Red, purple | Perrot’ ruby glass mid 17th century | [29] | Not studied | - |
| Enamel on glaze | French soft-paste porcelain and faience, >1st quarter of 18th century | [30,88] | Famille rose (Yongzheng reign 1722-1735) | [28,35] | |||
| Enamel on metal | Blois and Paris watches, >mid-17th century | [24] | Qianlong, F1467 | This work | |||
| Cassiterite | 635 | Glass | White | Late Roman Empire Perrot’ glass Mid-17th century | [29,51] | Not studied | - |
| Enamel on glaze | White yellow | Majolica 15th century, | - | huafalang, Kangxi, G5250 (light green) | [27,35] | ||
| Enamel on metal | White Yellow green | Limoges <16th century Blois and Paris watches, 17th century | [12,13,24] | Qianlong, F1467 | This work | ||
| Pb-(Sn,Zn)-Sb pyrochlore | 130–140 | Glass | Yellow | Roman times Perrot’ glass, 17th century | [29,51] | Not studied | - |
| (Enamel on) glaze | Yellow Green | Majolica 15th century | [53,54,55,56,57,58,59,60,61] | Qianlong, F1429C | This work,35 | ||
| Enamel on metal | Yellow (Green) | Limoges 16th century Blois and Paris watches, 17th century | [12,13,24] | Cloisonné: Kangxi reign F1448C Painted: Qianlong G5068 (18th c., Jingdezhen) G3361 (Kangxi?, Jingdezhen) | This work | ||
| Pb-(Sn,Zn)-Sb pyrochlore | ~140 + 510 | Glass | Yellow | Roman times Perrot’ glass, 17th century | [29,51] | Not studied | - |
| Enamel on glaze | Yellow | French soft-paste porcelain 18th century | [30] | huafalang: Kangxi G5250 | [27] | ||
| Enamel on metal | Yellow | Limoges 17th–18th century Blois and Paris watches, 18th century | [12,13,24] | Cloisonné: Qianlong F1467 | This work | ||
| As–O signature in blue | 815–820 | Glass | Blue | Perrot’ glass 17th century | [29] | Not studied | - |
| Enamel on glaze | Blue | St-Cloud, Paris Soft-paste porcelain 17th century | - | huafalang: Kangxi, G5250 | [27,30,43] | ||
| Enamel on metal | Blue | Limoges 17th century Blois and Paris watches, 17th century | [12,13,24] | Cloisonné: Qianlong F1501 | This work | ||
| Lead–arsenic apatite | 820, 780 | Glass | White | Lattimo (Venice, 17th century) | [57] | Not studied | - |
| Enamel on glaze | White | Not observed | - | G3361 (Kangxi?, Jingdezhen) | [27] | ||
| Enamel on metal | White | Limoges 19th century | [12,13] | Qianlong, F1440C | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Non-Invasive On-Site Raman Study of Pigments and Glassy Matrix of 17th–18th Century Painted Enamelled Chinese Metal Wares: Comparison with French Enamelling Technology. Coatings 2020, 10, 471. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10050471
Colomban P, Kırmızı B, Zhao B, Clais J-B, Yang Y, Droguet V. Non-Invasive On-Site Raman Study of Pigments and Glassy Matrix of 17th–18th Century Painted Enamelled Chinese Metal Wares: Comparison with French Enamelling Technology. Coatings. 2020; 10(5):471. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10050471
Chicago/Turabian StyleColomban, Philippe, Burcu Kırmızı, Bing Zhao, Jean-Baptiste Clais, Yong Yang, and Vincent Droguet. 2020. "Non-Invasive On-Site Raman Study of Pigments and Glassy Matrix of 17th–18th Century Painted Enamelled Chinese Metal Wares: Comparison with French Enamelling Technology" Coatings 10, no. 5: 471. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10050471