Mesoporous CoOx/C Nanocomposites Functionalized Electrochemical Sensor for Rapid and Continuous Detection of Nitrite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
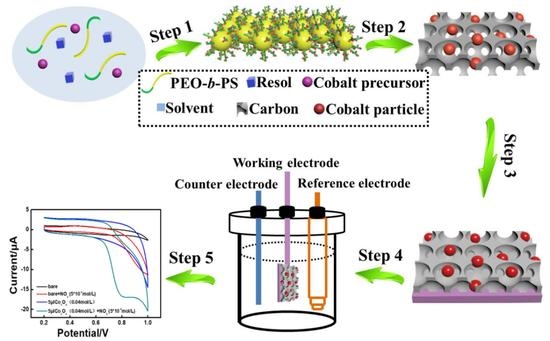
2.2. Synthesis of CoOx/C Nanocomposites
2.3. Preparation of the Modified Electrode
2.4. Characterization and Measurements
3. Results and Discussion
3.1. Characterization
3.2. Electrochemical and Catalytic Activity
3.3. Stability and Selectivity
3.4. Application of the Sensor in Practical Food Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Yu, L.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Palanisamy, S.; Yang, T. Single-crystalline SnS2 nano-hexagons based non-enzymatic electrochemical sensor for detection of carcinogenic nitrite in food samples. Sens. Actuators B Chem. 2020, 316, 128106. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, C.; Yue, G.; Yang, Z.; Wang, Y.; Rao, H.; Zhang, W.; Jin, B.; Wang, X. A highly selective chromogenic probe for the detection of nitrite in food samples. Food Chem. 2020, 317, 126361. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Deng, G.; Zou, C.; Xu, Y.; Cheng, W. Advances in Degradation Method for Nitrite in Vegetables. Hunan Agric. Sci. 2017, 7, 109–111. [Google Scholar]
- Li, X.; Meng, X.; Li, J. Research progress on method for nitrite abatement in pickles. J. Agric. Sci. Technol. 2012, 14, 90–95. [Google Scholar]
- Viuda-Martos, M.; Fernandez-Lopez, J.; Sayas-Barbera, E.; Sendra, E.; Navarro, C.; Perez-Alvarez, J. Citrus Co-Products as Technological Strategy to Reduce Residual Nitrite Content in Meat Products. J. Food Sci. 2009, 74, R93–R100. [Google Scholar] [CrossRef]
- Liu, D.; Wang, P.; Zhang, X.; Xu, X.; Wu, H.; Li, L. Characterization of Nitrite Degradation by Lactobacillus casei subsp rhamnosus LCR 6013. PLoS ONE 2014, 9, e93308. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Li, B.; Gao, X. Evaluating nitrite content changes in some Chinese home cooking with a newely-developed CDs diazotization spectrophotometry. Food Chem. 2020, 330, 127151. [Google Scholar] [CrossRef]
- Gill, A.; Zajda, J.; Meyerhoff, M. Comparison of electrochemical nitric oxide detection methods with chemiluminescence for measuring nitrite concentration in food samples. Anal. Chim. Acta 2019, 1077, 167–173. [Google Scholar] [CrossRef]
- Zhang, S.; Peng, R.; Jiang, R.; Chai, X.; Barnesc, D. A high-throughput headspace gas chromatographic technique for the determination of nitrite content in water samples. J. Chromatogr. A 2018, 1538, 104–107. [Google Scholar] [CrossRef]
- Wang, X.; Adams, E.; Schepdael, A. A fast and sensitive method for the determination of nitrite in human plasma by capillary electrophoresis with fluorescence detection. Talanta 2012, 97, 142–144. [Google Scholar] [CrossRef]
- Ferreira, F.T.; Mesquita, R.B.; Rangel, A.O. Microfluidic paper-based analytical devices for the determination of salivary NOx. In Proceedings of the XXIV Encontro Luso-Galego de Química, Porto, Portugal, 21–23 November 2018; p. 330. [Google Scholar]
- Maruo, Y.Y. Measurement of ambient ozone using newly developed porous glass sensor. Sens. Actuators B Chem. 2007, 126, 485–491. [Google Scholar] [CrossRef]
- Veiko, V.P.; Zakoldaev, R.A.; Sergeev, M.M.; Danilov, P.A.; Kudryashov, S.I.; Kostiuk, G.K.; Medvedev, O.S. Direct Laser Writing of Barriers with Controllable Permeability in Porous Glass. Opt. Express 2018, 26, 28150–28160. [Google Scholar] [CrossRef] [PubMed]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L. Analysis of food Additives. In Innovative Food Analysis; Galanakis, C., Ed.; Academic Press: Pittsburgh, PA, USA, 2021; Volume 7, pp. 157–180. [Google Scholar]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of nitrogen moieties in N-doped 3D-graphene nanosheets for oxygen electroreduction in acidic and alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Cai, Z.; Xiong, H.; Zhu, Z.; Huang, H.; Li, L.; Huang, Y.; Yu, X. Electrochemical synthesis of graphene/polypyrrole nanotube composites for multifunctional applications. Synth. Met. 2017, 227, 100–105. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shayeh, J.S.; Omidi, M.; Yazdian, F.; Alebouyeh, M.; Tayebi, L. A glassy carbon electrode modified with reduced graphene oxide and gold nanoparticles for electrochemical aptasensing of lipopolysaccharides from Escherichia coli bacteria. Microchim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B Chem. 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Ding, S.; Li, X.; Jiang, X.; Hu, Q.; Yan, Y.; Zheng, Q.; Lin, D. Core-shell nanostructured ZnO@CoS arrays as advanced electrode materials for high-performance supercapacitors. Electrochim. Acta 2009, 137, 710–714. [Google Scholar]
- Teymourian, H.; Salimi, A.; Khezrian, S. Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform. Biosens. Bioelectron. 2013, 49, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haldorai, Y.; Kim, J.; Vilian, A.; Heo, N.; Huh, Y.; Han, Y. An enzyme-free electrochemical sensor based on reduced graphene oxide/Co3O4 nanospindle composite for sensitive detection of nitrite. Sens. Actuators B Chem. 2020, 227, 92–99. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, J.; Zhao, D. Cobalt monoxide nanoparticles modified glassy carbon electrodes as a sensor for determination of nitrite. Adv. Mater. Res. 2015, 1120–1121, 291–298. [Google Scholar] [CrossRef]
- Liu, H.; Guo, K.; Lv, J.; Gao, Y.; Duan, C.; Deng, L.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of horseradish peroxidase immobilized on porous Co3O4 nanosheets and reduced graphene oxide composite modified electrode. Sens. Actuators B Chem. 2017, 238, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhao, D. Ordered mesoporous materials as adsorbents. Chem. Commun. 2011, 47, 3332–3338. [Google Scholar] [CrossRef]
- Wu, Z.; Li, W.; Webley, P.; Zhao, D. General and controllable synthesis of novel mesoporous magnetic iron oxide@carbon encapsulates for efficient arsenic removal. Adv. Mater. 2020, 24, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schüth, F. Controllable synthesis of mesoporous peapod-like Co3O4@Carbon nanotube arrays for high-performance lithium-ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 7060–7064. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, T.; Pan, X.; Sun, X.; Fan, X.; Guo, Y.; Xue, H.; He, J. Synthesis and electrochemical characterization of n-doped partially graphitized ordered mesoporous carbon–Co composite. J. Phys. Chem. C 2013, 117, 16896–16906. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Qiao, M.; Wei, J.; Yue, Q.; Wang, C.; Deng, Y.; Kaliaguine, S.; Zhao, D. A general chelate-assisted Co-assembly to metallic nanoparticles-incorporated ordered mesoporous carbon catalysts for fischer–tropsch synthesis. J. Am. Chem. Soc. 2020, 134, 17653–17660. [Google Scholar] [CrossRef]
- Mohamed, M.; Atayde, E.; Matsagar, B.; Na, J.; Yamauchi, Y.; Wu, K.; Kuo, K. Construction hierarchically mesoporous/microporous materials based on block copolymer and covalentorganic framework. J. Taiwan Inst. Chem. E 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Luo, W.; Li, Y.; Dong, J.; Wei, J.; Xu, J.; Deng, Y.; Zhao, D. A Resol-assisted Co-Assembly approach to crystalline mesoporous niobia spheres for electrochemical biosensing. Angew. Chem. Int. Ed. 2013, 52, 10505–10510. [Google Scholar] [CrossRef]
- Xiong, H.; Zhou, H.; Qi, C.; Liu, Z.; Zhang, L.; Zhang, L.; Qiao, Z. Polymer-oriented evaporation induced self-assembly strategy to synthesize highly crystalline mesoporous metal oxides. Chem. Eng. J. 2020, 398, 125527. [Google Scholar] [CrossRef]
- Li, Y.; Luo, W.; Qin, N.; Dong, J.; Wei, J.; Li, W.; Feng, S.; Chen, J.; Xu, J.; Elzatahry, A.; et al. Highly ordered mesoporous tungsten oxides with a large pore size and crystalline framework for H2S sensing. Angew. Chem. Int. Ed. 2014, 53, 9035–9040. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Gu, D.; Wang, S.; She, L.; Che, R.; Wang, Z.; Tu, B.; Xie, S.; Zhao, D. Ligand-assisted assembly approach to synthesize large-pore ordered mesoporous titania with thermally stable and crystalline framework. Adv. Energy Mater. 2011, 1, 241–248. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Luo, W.; Ren, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Controlled synthesis of ordered mesoporous carbon-cobalt oxide nanocomposites with large mesopores and graphitic walls. Chem. Mater. 2016, 28, 7773–7780. [Google Scholar] [CrossRef]
- Bresser, D.; Paillard, E.; Niehoff, P.; Krueger, S.; Mueller, F.; Winter, M.; Passerini, S. Challenges of “going nano”: Enhanced electrochemical performance of cobalt oxide nanoparticles by carbothermal reduction and in situ carbon coating. Chem. Phys. Chem. 2014, 15, 2177–2185. [Google Scholar] [CrossRef]
- Jin, H.; Wang, J.; Su, D.; Wei, Z.; Pang, Z.; Wang, Y. In situ cobal-cobalt oxide/n-doped carbon hybrids as superior bifunctional electrocatalysts for hydrogen and oxygen evolution. J. Am. Chem. Soc. 2015, 137, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Mao, S.; Su, D.; Jin, H.; Wang, Y.; Xu, F.; Li, H.; Wang, Y. In situ-generated Co0-Co3O4/n-doped carbon nanotubes hybrids as efficient and chemoselective catalysts for hydrogenation of nitroarenes. ACS Catal. 2015, 5, 4783–4789. [Google Scholar] [CrossRef]
- GB 5009.33-2016. Food Safety National Standard-Determination of Nitrite and Nitrate in Food; The Standardization Administration of the People′s Republic of China: Beijing, China, 2016. [Google Scholar]






| Sample No. | NO2− Added (mg/kg) | NO2− Found (mg/kg) | Recovery (%) |
|---|---|---|---|
| 1 | 5.00 | 17.75 | 101.8 |
| 2 | 10.00 | 22.43 | 97.7 |
| 3 | 15.00 | 27.63 | 99.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Xie, S.; Zhu, J.; Liu, H.; Zhao, Y.; Ni, T.; Wu, L.; Zhu, Y. Mesoporous CoOx/C Nanocomposites Functionalized Electrochemical Sensor for Rapid and Continuous Detection of Nitrite. Coatings 2021, 11, 596. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11050596
Dong X, Xie S, Zhu J, Liu H, Zhao Y, Ni T, Wu L, Zhu Y. Mesoporous CoOx/C Nanocomposites Functionalized Electrochemical Sensor for Rapid and Continuous Detection of Nitrite. Coatings. 2021; 11(5):596. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11050596
Chicago/Turabian StyleDong, Xuhua, Siqi Xie, Jingyang Zhu, Haiquan Liu, Yong Zhao, Tianjun Ni, Long Wu, and Yongheng Zhu. 2021. "Mesoporous CoOx/C Nanocomposites Functionalized Electrochemical Sensor for Rapid and Continuous Detection of Nitrite" Coatings 11, no. 5: 596. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings11050596