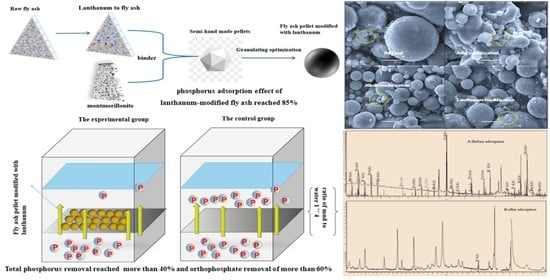
Control of Endogenous Phosphorus Release at the Sediment–Water Interface by Lanthanum-Modified Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Modified Fly Ash
2.2.2. Adsorption Experiment
2.2.3. Optimal Dosage Test
2.2.4. Optimal pH and Temperature Experiments
2.3. Fly Ash Granulation Experiment
2.3.1. Process Flow
2.3.2. Orthogonal Experimental Design
2.4. Study of Lanthanum Modified Fly Ash Pellets to Control Endogenous Phosphorus Release at the Sediment–Water Interface
2.5. Raw Water Quality
2.6. Analytical and Characterization Methods
3. Results and Discussion
3.1. Study of Adsorption Efficiency of Modified Fly Ash
3.1.1. Adsorption Effect and Characterization of Different Modified Fly Ash Materials
3.1.2. Experimental Investigation of the Adsorption Efficiency Resulting from Lanthanum Modification
3.2. Orthogonal Analysis of Modified Fly Ash Granulation
3.3. In Situ Control of Endogenous Phosphorus at the Sediment–Water Interface by Modified Fly Ash Pellets
3.4. Study of Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
References
- Ming, J.J.; Shu, W.D.; Fu, W.; Yan, R.; Yi, Z.C. Content and solubility of phosphorus in atmospheric particulate matter. Min. Res. Geochem. B 2022, 41, 7+70–80. [Google Scholar]
- Ting, W.; Hong, J.W.; Long, Z.Q.; Qian, G.L.; Si, J.Z.; Guang, M.Z. Research progress of metal (hydrogen) oxide-based phosphorus removal adsorbents. Water Treat. Technol. 2021, 47, 12–17+23. [Google Scholar]
- Zhang, B.; Lin, N.; Chen, X. Nonlinear Water Quality Response to Numerical Simulation of In Situ Phosphorus Control Approaches. Water 2021, 13, 725. [Google Scholar] [CrossRef]
- Fu, Z. Control Effects and Mechanisms of In Situ Passivation of Lake Sediments on Endogenous Phosphorus. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2019. [Google Scholar]
- Xiurong, H. Eutrophication of water bodies and its prevention and control measures. Lea. Mak. Environ. Technol. 2021, 2, 107–108. [Google Scholar]
- Zhenghai, J.; Cheng, Q.C.; Shuhang, W. Phosphorus sorption characteristics and release risk of cyanobacteria from lake and bay sediments during the overwintering period. Environ. Sci. 2022, 43, 1976–1987. [Google Scholar]
- Xinyu, M.; Pan, Y.; Man, Z. Progress of phosphorus passivation materials for lake sediments. Lake Sci. 2021, 34, 1–17. [Google Scholar]
- Ren, Q.Q.; Tang, W.Y.; Ying, P.; Yin, H.B. Effect of lanthanum modified bentonite on the control of endogenous phosphorus in bottom sediment. China Environ. Sci. 2021, 41, 199–206. [Google Scholar]
- Oldenborg, K.A.; Steinman, A.D. Impact of sediment dredging on sediment phosphorus flux in a restored riparian wetland. Sci. Total Environ. 2019, 650, 1969–1979. [Google Scholar] [CrossRef]
- Yang, M.; Lin, J.; Zhan, Y. Adsorption of phosphate from water on lake sediments amended with zirconium-modified zeolites in batch mode. Ecol. Eng. 2014, 71, 223–233. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Tabassum, S. Effect of calcium silicate hydrates (CSH) on phosphorus immobilization and speciation in shallow lake sediment. Chem. Eng. J. 2017, 317, 844–853. [Google Scholar] [CrossRef]
- Bao, T.; Chen, T.H.; Qing, C.; Jin Xie, J.; Frost, R.L. Development and application of Palygorskite porous ceramsite in a biological aerated filter (BAF). Desalin. Water Treat. 2016, 57, 1790–1803. [Google Scholar] [CrossRef] [Green Version]
- Fang, T.; Bao, S.; Sima, X. Study on the application of integrated eco-engineering in purifying eutrophic river waters. Ecol. Eng. 2016, 94, 320–328. [Google Scholar] [CrossRef]
- Recknagel, F.; Hosomi, M.; Fukushima, T. Short-and long-term control of external and internal phosphorus loads in lakes—A scenario analysis. Water Res. 1995, 29, 1767–1779. [Google Scholar] [CrossRef]
- Yin, H.; Yang, C.; Yang, P. Contrasting effects and mode of dredging and in situ adsorbent amendment for the control of sediment internal phosphorus loading in eutrophic lakes. Water Res. 2021, 189, 116644. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, S.; Zhu, B. Al-PHOSLOCK thin-layer capping to control phosphorus release from sediment: Effect of hydraulic retention time and phosphorus migration/transformation mechanism. J. Soil Sediments 2021, 21, 2474–2482. [Google Scholar] [CrossRef]
- Qiuzhe, C.; Lijin, Z.; Yifei, G. Effect and mechanism of magnesium-lanthanum composites and their combination with potassium persulfate on the control of endogenous phosphorus release in water bodies. Environ. Chem. 2021, 40, 2841–2853. [Google Scholar]
- Shutong, C.; Dapeng, L.; Chutian, X. Influence of substrate microenvironment on endogenous phosphorus release under cover conditions. Environ. Sci. 2021, 42, 2848–2855. [Google Scholar]
- Xiaoyun, B.; Jianwei, L.; Yanhui, Z. Controlling endogenous phosphorus release from water bodies using iron-modified calcite as an active cover material. Environ. Sci. 2020, 41, 1296–1307. [Google Scholar]
- Tang, M.; Deng, Q.; Cao, X. Mechanisms and risks of joint control of nitrogen and phosphorus through sediment capping technology in a pilot-scale study. J Soils Sediments 2021, 21, 3427–3437. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liu, S. Three kinds of active thin-layer capping materials for reducing the phosphorus load in eutrophic water body: Comparison in dynamic experiment. Environ. Sci. Pollut. Res. Int. 2021, 29, 16427–16435. [Google Scholar] [CrossRef]
- Gao, C.; Fan, J.; Zhang, X. Sediment metals adhering to biochar enhanced phosphorus adsorption in sediment capping. Water Sci. Technol. 2021, 84, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.C.; Zhao, Q.J.; Yu, X. Study on bioaugmented phosphorus recovery technology for wastewater. Appl. Chem. 2021, 50, 1377–1381. [Google Scholar]
- Zhou, J.; Fu, Y.; Pan, S. The use of modified coal gangue for the remediation and removal of phosphorus in an enclosed water area. Clean Technol. Environ. Policy 2021, 23, 1327–1339. [Google Scholar] [CrossRef]
- Gu, S.; Fu, B.; Ahn, J.W.; Fang, B. Mechanism for phosphorus removal from wastewater with fly ash of municipal solid waste incineration, Seoul, Korea. J. Clean. Prod. 2021, 280, 124430. [Google Scholar] [CrossRef]
- Jin, H.; Lin, L.; Meng, X.; Wang, L.; Huang, Z.; Liu, M.; Crittenden, J.C. A novel lanthanum-modified copper tailings adsorbent for phosphate removal from water. Chemosphere 2021, 281, 130779. [Google Scholar] [CrossRef]
- Xia, W.J.; Guo, L.X.; Yu, L.Q.; Xiong, J.R.; Zhu, X.Y.; Jin, R.C. Phosphorus removal from diluted wastewaters using a La/C nanocomposite-doped membrane with adsorption-filtration dual functions. Chem. Eng. J. 2021, 405, 126924. [Google Scholar] [CrossRef]
- Bormans, M.; Maršálek, B.; Jančula, D. Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms: A review. Aquat. Ecol. 2015, 50, 407–422. [Google Scholar] [CrossRef]
- Park, J.H.; Hwang, S.W.; Lee, S.L.; Lee, J.H.; Seo, D.C. Sorption behavior of phosphate by fly ash discharged from biomass thermal power plant. Appl. Biol. Chem. 2021, 64, 43. [Google Scholar] [CrossRef]
- Xu, R.; Lyu, T.; Wang, L. Utilization of coal fly ash waste for effective recapture of phosphorus from waters. Chemosphere 2021, 287 Pt 4, 132431. [Google Scholar] [CrossRef]
- Shi, J.; Han, F.; Qiu, L. Adsorption performance of compound modified fly ash on phosphorus. Chem. Environ. Prot. 2020, 40, 180–185. [Google Scholar]
- Jia, X.C.; Liu, Y.W.; Kun, Z. Progress of fly ash-based zeolite treatment of nitrogen and phosphorus wastewater. Silic. Bul. 2021, 40, 2622–2630. [Google Scholar]
- Yan, P.J.; Xiu, P.J.; Cheng, J. Preparation of modified fly ash and its application progress in the treatment of phosphorus-containing wastewater. Silic. Bul. 2015, 34, 1921–1925. [Google Scholar]
- Zhi, C.L.; Xiao, Y.S.; Yan, G.L. Lanthanum modified fly ash and its effect on denitrification and phosphorus removal. New Chem. Mat. 2018, 46, 205–208. [Google Scholar]
- Liu, M.; Wang, C.; Guo, J.; Zhang, L. Removal of phosphate from wastewater by lanthanum modified bio-ceramisite. J. Environ. Chem. Eng. 2021, 9, 106123. [Google Scholar] [CrossRef]
- Long, S.L.; Miao, L.; Qian, W. Study on the adsorption of phosphorus-containing wastewater by lanthanum-modified fly ash geopolymer foam. Min. Metall. Eng. 2020, 40, 103–107. [Google Scholar]
- Copetti, D.; Finsterle, K.; Marziali, L.; Stefani, F.; Tartari, G.; Douglas, G.; Lürling, M. Eutrophication management in surface waters using lanthanum modified bentonite: A review. Water Res. 2016, 97, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Y.; Sun, Y. La(OH)3 Loaded Magnetic Mesoporous Nanospheres with Highly Efficient Phosphate Removal Properties and Superior pH Stability. Chem. Eng. J. 2018, 360, 342–348. [Google Scholar] [CrossRef]
- You, K.; Yang, W.; Song, P. Lanthanum-modified magnetic oyster shell and its use for enhancing phosphate removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127897. [Google Scholar] [CrossRef]
- Cervelli, M.J.; Shaman, A.; Meade, A.; Carroll, R.; McDonald, S.P. Effect of gastric acid suppression with pantoprazole on the efficacy of calcium carbonate as a phosphate binder in haemodialysis patients. Nephrology 2012, 17, 458–465. [Google Scholar] [CrossRef]
- Yang, Y.; Bykadi, S.; Carlin, A.S.; Shah, R.B.; Lawrence, X.Y. Comparative evaluation of the in vitro efficacy of lanthanum carbonate chewable tablets. J. Pharm. Sci. 2013, 102, 1370–1381. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Lu, S. Removal and recovery of phosphate from water by lanthanum hydroxide materials. Chem. Eng. J. 2014, 254, 163–170. [Google Scholar] [CrossRef]
- Zhan, Y.; Chang, M.; Lin, J. Suppression of phosphorus release from sediment using lanthanum carbonate as amendment. Environ. Sci. Pollut. Res. 2021, 28, 3280–3295. [Google Scholar] [CrossRef] [PubMed]
- LiMei, M.; TuJin, W.; Lin, C. Preparation and Phosphorus and Algae Removal of Polyaluminum Chloride-Lanthanum Modified Bentonite. Environ. Sci. Res. 2021, 1–12. [Google Scholar]
- Kong, M.; Liu, F.; Zhang, W.; Xu, X.; Chao, J.; Wang, P.; Gao, Y. High resolution investigation of pH on control of mobile and organic phosphorus and associated release kinetics following lanthanum modified bentonite (LMB) amendment at the water-sediment interface. J. Clean. Prod. 2021, 295, 126148. [Google Scholar] [CrossRef]
- Yang, Y.; Jian, W.L.; Yan, H.Z.; Si, Q.H.; Xiao, L.W.; Yan, W.; Yu, Y.Z.; Ying, L.; Peng, X.L. Effects of zirconium-modified zeolite addition on phosphorus migration transformation in river bottom sediment under static and hydrodynamic disturbance conditions. Environ. Sci. 2019, 40, 1337–1346. [Google Scholar]
- Razanajatovo, M.R.; Gao, W.; Song, Y.; Zhao, X.; Sun, Q.; Zhang, Q. Selective adsorption of phosphate in water using lanthanum-based nanomaterials: A critical review. Chin. Chem. Lett. 2021, 32, 2637–2647. [Google Scholar] [CrossRef]
- Ifthikar, J.; Zhao, M.; Sellaoui, L.; Oyekunle, D.T.; Li, J.; Zeng, Z.; Wang, S.; Wu, B.; Wang, J.; Chen, Z. Phosphate sequestration by lanthanum-layered rare earth hydroxides through multiple mechanisms while avoiding the attenuation effect from sediment particles in lake water. Sci. Total Environ. 2022, 830, 154786. [Google Scholar] [CrossRef]
- He, K.; Chen, Y.; Tang, Z. Removal of heavy metal ions from aqueous solution by zeolite synthesized from fly ash. Environ. Sci. Pollut. Res. 2016, 23, 2778–2788. [Google Scholar] [CrossRef]
- Deng, X.; Qi, L.; Zhang, Y. Experimental Study on Adsorption of Hexavalent Chromium with Microwave-Assisted Alkali Modified Fly Ash. Water Air Soil Pollut. 2018, 229, 18. [Google Scholar] [CrossRef]
- Mario, N.M.; Sandra, S.; Jelica, Z. Kinetic studies of cobalt ion removal from aqueous solutions using fly ash-based geopolymer and zeolite NaX as sorbents. Sep. Sci. Technol. 2016, 51, 2868–2875. [Google Scholar]
- Yang, Z.H.; Zhang, Z.H.; Li, X.T.; Xu, S.X.; Mi, X.Y. Research on the application of binder in converter slag making agent. Min. Metal. Eng. 2019, 39, 99–102. [Google Scholar]
- Miao, X.G.; Shen, L.; Xu, L.H.; Zhou, X.P.; Liu, D.G. Preparation and Properties of Iron-sealed Glass Granulated Powder. Gla Ena 2016, 44, 10–18. [Google Scholar]
- Haghseresht, F.; Wang, S.; Do, D.D. A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters. Appl. Clay Sci. 2009, 46, 369–375. [Google Scholar] [CrossRef]
- Zhou, Z.; Lin, C.; Li, S.; Liu, S.; Li, F.; Yuan, B. Four kinds of capping materials for controlling phosphorus and nitrogen release from contaminated sediment using a static simulation experiment. Front. Environ. Sci. Eng. 2022, 16, 29. [Google Scholar] [CrossRef]
- Zhu, B.; Li, S.; Lin, C.; Liu, S.; Li, F.; Zhou, Z. The effect of secondary capping on the control of phosphorus release from sediment by activated thin-layer capping with Al-PIA. Environ. Sci. Pollut. Res. 2021, 28, 18062–18069. [Google Scholar] [CrossRef]
- Sun, C.; Xiong, W.; Zhang, W.; Liu, Z.; Li, Y.; Zhou, X.; Niu, L.; Zhang, H.; Wang, L. New insights into identifying sediment phosphorus sources in river-lake coupled system: A framework for optimizing microbial community fingerprints. Environ. Res. 2022, 209, 112854. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wu, H. Insight into simultaneous selective removal of nitrogen and phosphorus species by lanthanum-modified porous polymer: Performance, mechanism and application. Chem. Eng. J. 2021, 415, 129026. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Q.; Li, S.; Li, F.; Zou, J.; Liao, X.; Yuan, B.; Sun, W. Characterizing the correlation between dephosphorization and solution pH in a calcined water treatment plant sludge. Environ. Sci. Pollut. Res. 2018, 25, 18510–18518. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, P.; Cui, R. Electrical conductivity and dissolved oxygen as predictors of nitrate concentrations in shallow groundwater in Erhai Lake region. Sci. Total Environ. 2022, 802, 149879. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yu, W. Lanthanum-based adsorbents for phosphate reutilization: Interference factors, adsorbent regeneration, and research gaps. Sustain. Horiz. 2022, 1, 100011. [Google Scholar] [CrossRef]
















| Compound | SiO2 | Al2O3 | Cr2O3 | Fe2O3 | MnO | CaO | TiO2 | K2O | FeS | SO3 | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Original fly ash | 36.3% | 13.0% | 7.0% | 3.6% | 2.3% | 2.0% | 4.9% | 5.0% | 3.5% | 15.4% | 3.9% |
| TP (mg/L) | SRP (mg/L) | TN (mg/L) | |
|---|---|---|---|
| Overlying water | 0.69 | 0.39 | 2.53 |
| Original Fly Ash | Lanthanum-Modified Fly Ash | Magnification (Lanthanum/Original) | |
|---|---|---|---|
| Specific surface area m2/g | 2.21 | 6.21 | 3 |
| Pores nm * | / | 2~25 | / |
| Average pore size nm ** | / | 8.5 | / |
| Total pore volume% | 12.80 | 89.63 | 7 |
| Compound | Original Fly Ash | Acid-Modified | Alkali-Modified | Lanthanum-Modified |
|---|---|---|---|---|
| SiO2 | 36 | 27.6 | 29 | 21.3 |
| Al2O3 | 17 | 15.1 | 15.7 | 16.5 |
| Cr2O3 | 7 | 8 | 8.1 | 8.5 |
| Fe2O3 | 5.6 | 4.6 | 4.9 | 5.2 |
| MnO | 2.3 | 3.6 | 3.4 | 4.2 |
| CaO | 5.0 | 2.9 | 3.5 | 2.7 |
| MgO | 3.1 | 5.7 | 5.8 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Liu, G.; Chai, B.; Lei, X.; He, L.; Cheng, S.; Wang, Y.; Chen, W.; Li, S.; Chen, L.; et al. Control of Endogenous Phosphorus Release at the Sediment–Water Interface by Lanthanum-Modified Fly Ash. Coatings 2022, 12, 719. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12060719
Pan Y, Liu G, Chai B, Lei X, He L, Cheng S, Wang Y, Chen W, Li S, Chen L, et al. Control of Endogenous Phosphorus Release at the Sediment–Water Interface by Lanthanum-Modified Fly Ash. Coatings. 2022; 12(6):719. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12060719
Chicago/Turabian StylePan, Ying, Gang Liu, Beibei Chai, Xiaohui Lei, Lixin He, Shuailong Cheng, Yijie Wang, Wenlong Chen, Simin Li, Liang Chen, and et al. 2022. "Control of Endogenous Phosphorus Release at the Sediment–Water Interface by Lanthanum-Modified Fly Ash" Coatings 12, no. 6: 719. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings12060719