A Comparative Study of the Effect of Field Retting Time on the Properties of Hemp Fibres Harvested at Different Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.1.1. Cultivation and Sampling
2.1.2. Weather Conditions
2.2. Experimental Methods
2.2.1. Visual Aspect
2.2.2. Microscopic Observations
2.2.3. Biochemical Analysis
2.2.4. X-Ray Diffraction
2.2.5. Thermogravimetric Analysis
2.2.6. Tensile Test of Fibre Bundles
3. Results and Discussion
3.1. Colour Change
3.2. Biochemical Composition
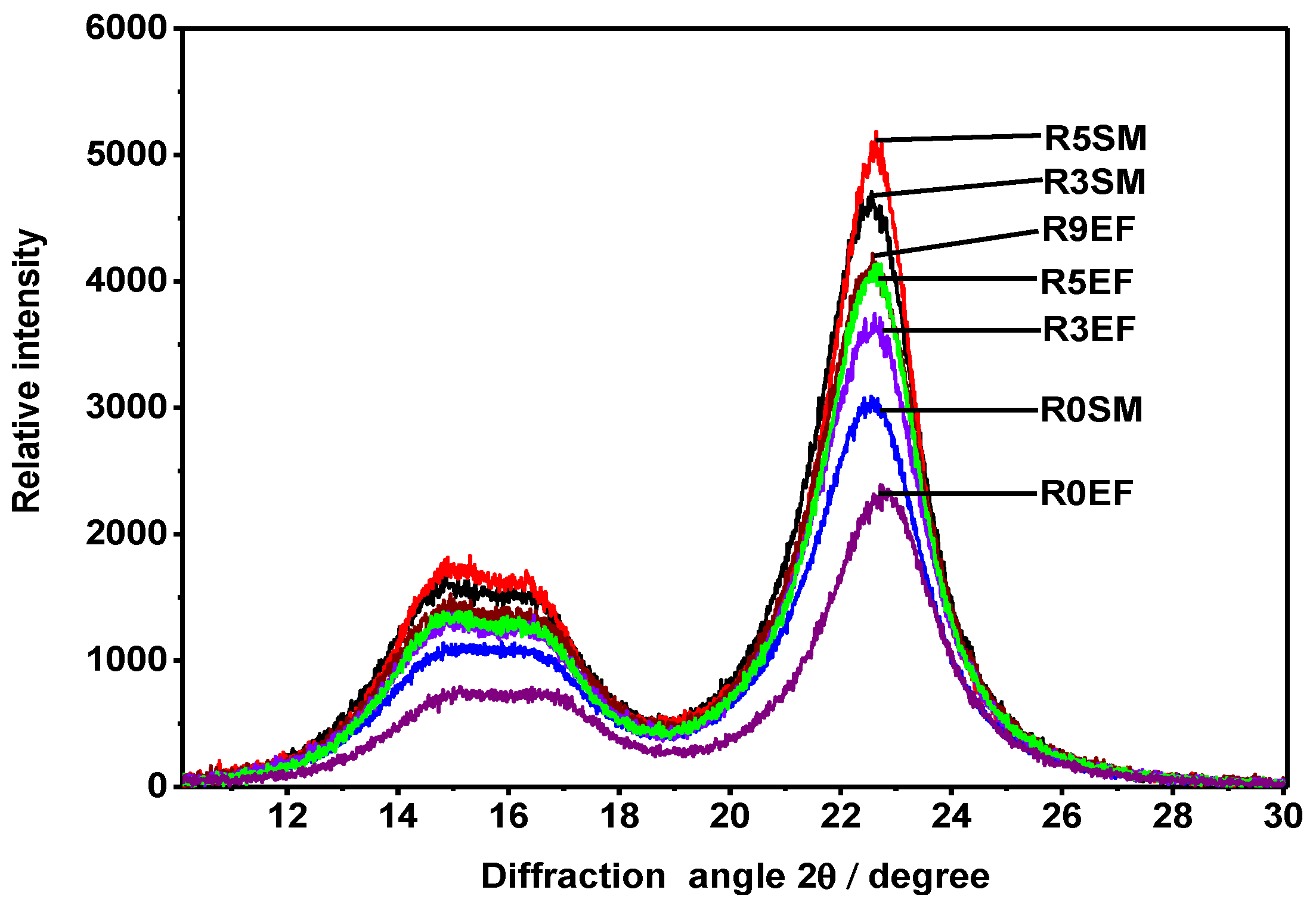
3.3. Cellulose Crystallinity
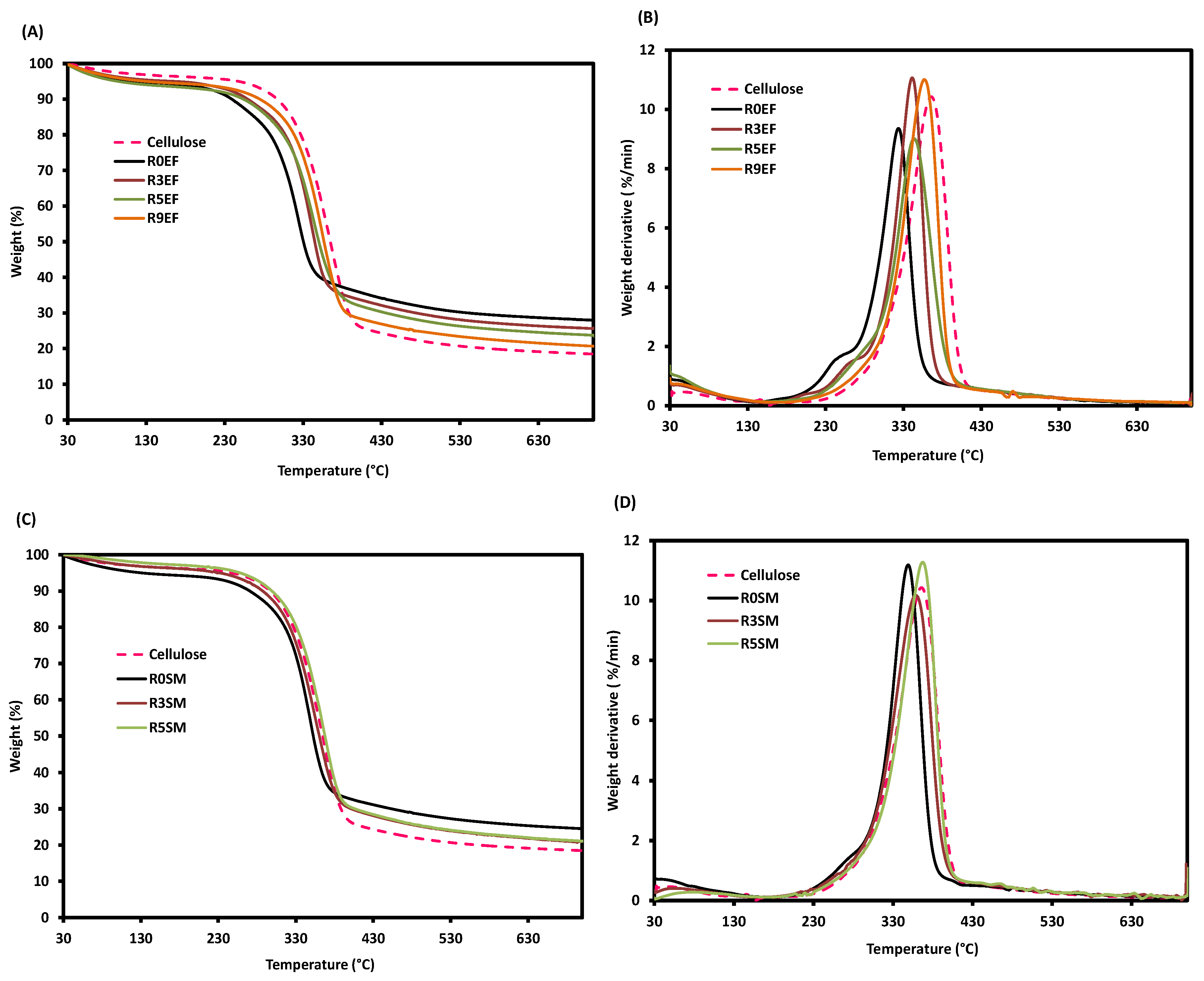
3.4. Thermal Stability
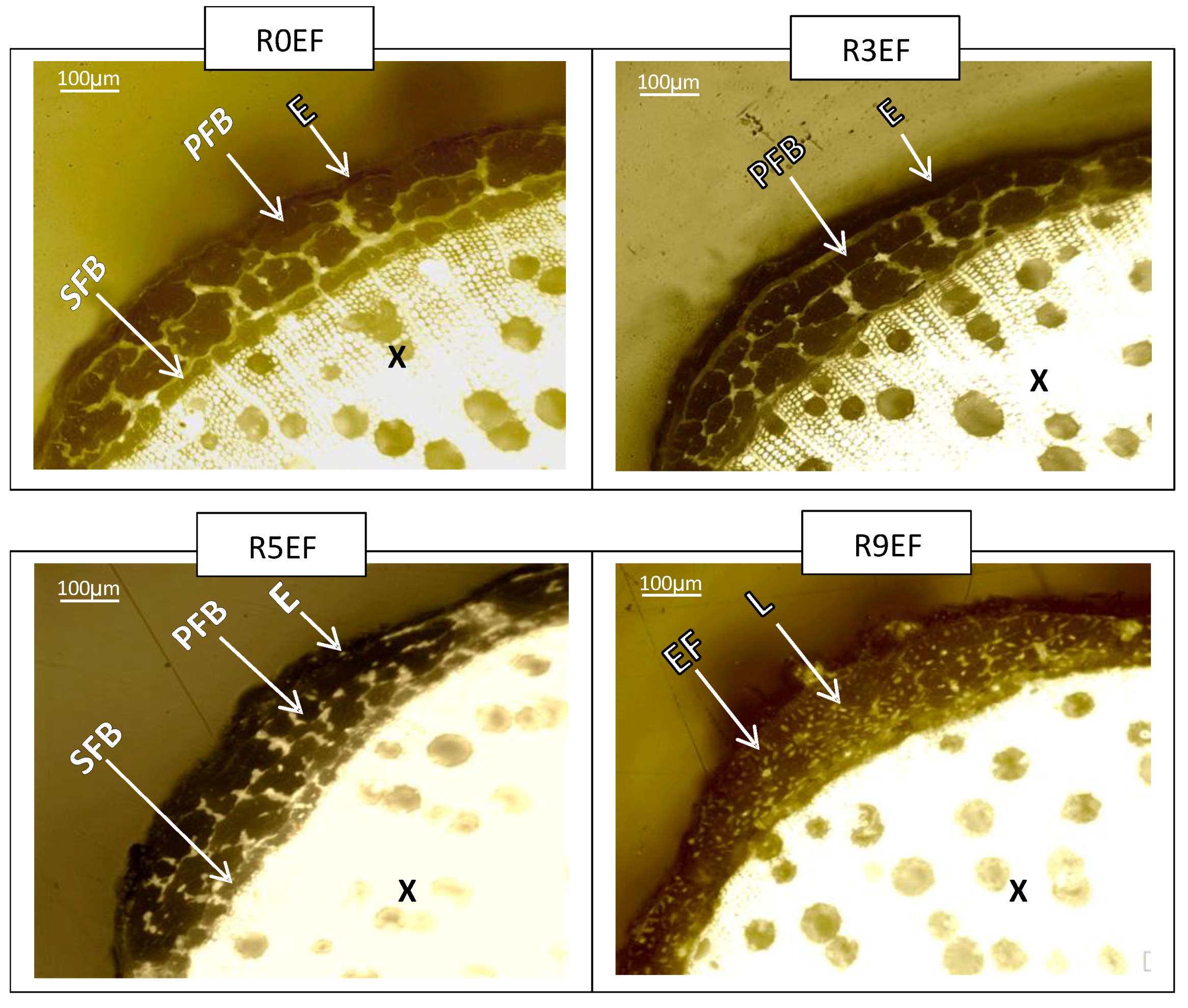
3.5. Morphology
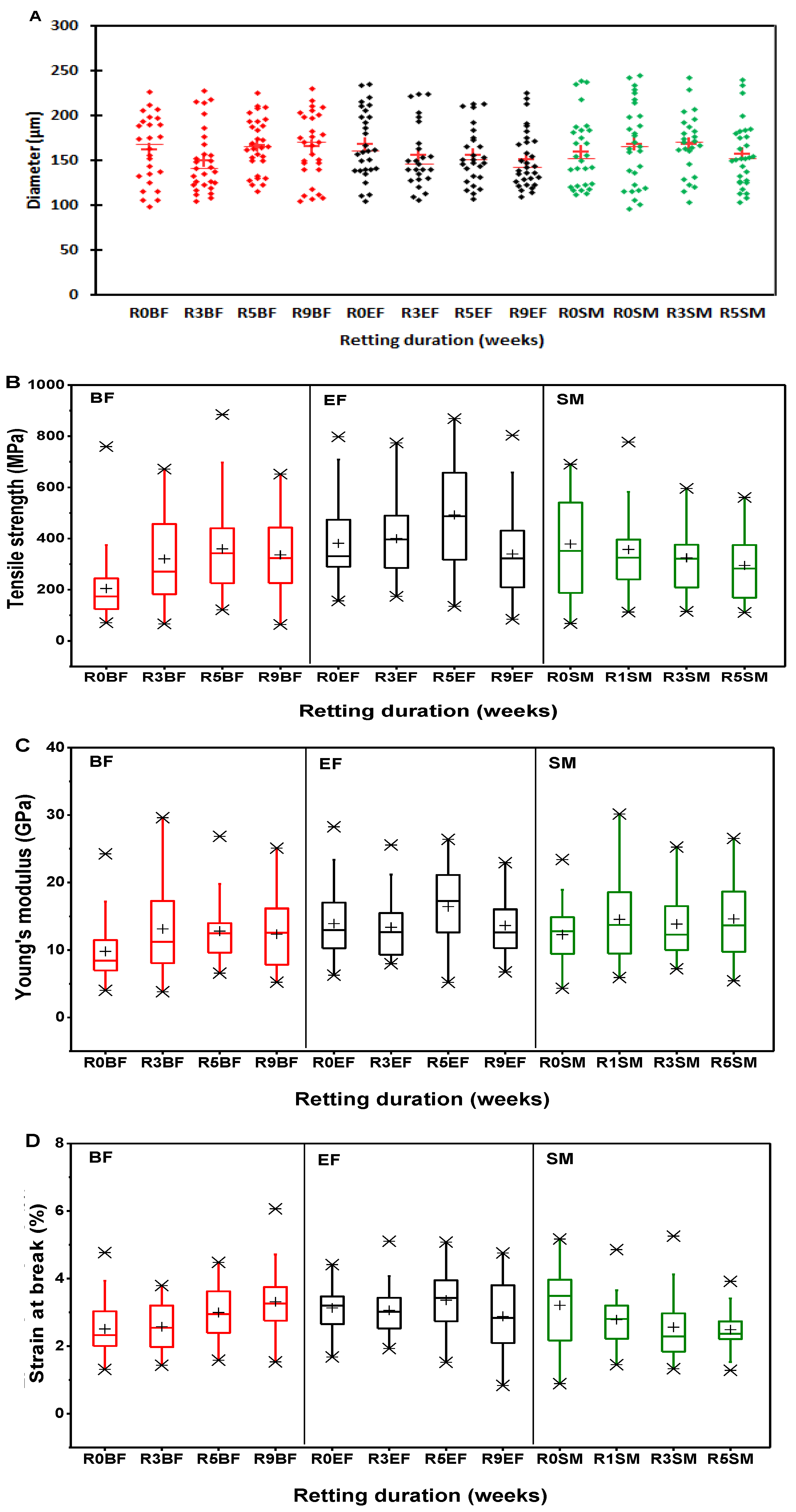
3.6. Tensile Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duval, A.; Bourmaud, A.; Augier, L.; Baley, C. Influence of the sampling area of the stem on the mechanical properties of hemp fibers. Mater. Let. 2011, 65, 797–800. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites: How can we best define the diversity and specificities of plant cell walls? Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Islam, M.S.; Pickering, K.L.; Foreman, N.J. Influence of alkali fiber treatment and fiber processing on the mechanical properties of hemp/epoxy composites. J. Appl. Polym. Sci. 2010, 21, 449–456. [Google Scholar] [CrossRef]
- Stamboulis, A.; Baillie, C.A.; Peijs, T. Effects of environmental conditions on mechanical and physical proprties of flax fibers. Compos. Part A 2001, 32, 1105–1115. [Google Scholar] [CrossRef]
- Aziz, S.H.; Ansell, M.P. The effect of alkalization and fibre alignment on the mechanical and thermal properties of kenaf and hemp bast fibre composites: Part 1-polyester resin matrix. Compos. Sci. Technol. 2004, 64, 1219–1230. [Google Scholar] [CrossRef]
- Mazian, B.; Bergeret, A.; Benezet, J.-C.; Malhautier, L. Impact of field retting and accelerated retting performed in a lab-scale pilot unit on the properties of hemp fibres/polypropylene biocomposites. Ind. Crops Prod. 2020, 143, 111912. [Google Scholar] [CrossRef]
- Marrot, L.; Lefeuvre, A.; Pontoire, B.; Bourmaud, A.; Baley, C. Analysis of the hemp fiber mechanical properties and their scattering (Fedora 17). Ind. Crops Prod. 2013, 51, 317–327. [Google Scholar] [CrossRef]
- Van der Werf, H.M.G.; Wijlhuizen, M.; de Schutter, J.A.A. Plant density and self-thinning affect yield and quality of fibre hemp (Cannabis sativa L.). Fields Crops Res. 1995, 40, 153–164. [Google Scholar] [CrossRef]
- Mediavilla, V.; Leupin, M.; Keller, A. Influence of the growth stage of industrial hemp on the yield formation in relation to certain fibre quality traits. Ind. Crops Prod. 2001, 13, 49–56. [Google Scholar] [CrossRef]
- Goudenhooft, C.; Siniscalco, D.; Arnould, O.; Bourmaud, A.; Sire, O.; Gorshkova, T.; Baley, C. Investigation of the mechanical properties of flax cell walls during plant development: The relation between performance and cell wall structure. Fibers 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Martin, N.; Mouret, N.; Davies, P.; Baley, C. Influence of the degree of retting of flax fibers on the tensile properties of single fibers and short fiber/polypropylene composites. Ind. Crops Prod. 2013, 49, 755–767. [Google Scholar] [CrossRef] [Green Version]
- Placet, V.; Day, A.; Beaugrand, J. The influence of unintended field retting on the physicochemical and mechanical properties of industrial hemp bast fibres. J. Mater. Sci. 2017, 52, 5759–5777. [Google Scholar] [CrossRef]
- Liu, M.; Fernando, D.; Daniel, G.; Madsen, B.; Meyer, A.S.; Ale, M.T.; Thygesen, A. Effect of harvest time and field retting duration on the chemical composition, morphology and mechanical properties of hemp fibers. Ind. Crops Prod. 2015, 69, 29–39. [Google Scholar] [CrossRef]
- Hearle, J.W. The fine structure of fibers and crystalline. J. Appl. Polym. Sci. 1963, 7, 1175–1192. [Google Scholar] [CrossRef]
- Crônier, D.; Monties, B.; Chabbert, B.R. Structure and chemical composition of bast fibers isolated from developing hemp stem. J. Agric. Food Chem. 2005, 53, 8279–8289. [Google Scholar] [CrossRef]
- Li, Y.; Pickering, K.L.; Farrell, R.L. Analysis of green hemp fibre reinforced composites using bag retting and white rot fungal treatments. Ind. Crops Prod. 2009, 29, 420–426. [Google Scholar] [CrossRef]
- Sisti, L.; Totaro, G.; Vannini, M.; Fabbri, P.; Kalia, S.; Zatta, A.; Celli, A. Evaluation of the retting process as a pre-treatment of vegetable fibers for the preparation of high-performance polymer biocomposites. Ind. Crops Prod. 2016, 81, 56–65. [Google Scholar] [CrossRef]
- Paridah, M.T.; Basher, A.B.; SaifulAzry, S.; Ahmed, Z. Retting process of some bast plant fibres and its effect on fibre quality: A review. BioResources 2011, 6, 5260–5281. [Google Scholar]
- Bacci, L.; Di Lonardo, S.; Albanese, L.; Mastromei, G.; Perito, B. Effect of different extraction methods on fiber quality of nettle (Urtica dioica L.). Text. Res. J. 2011, 81, 827–837. [Google Scholar] [CrossRef]
- Akin, D.E.; Condon, B.; Sohn, M.; Foulk, J.A.; Dodd, R.B.; Rigsby, L.L. Optimization for enzyme-retting of flax with pectate lyase. Ind. Crops Prod. 2007, 25, 136–146. [Google Scholar] [CrossRef]
- Henriksson, G.; Akin, D.E.; Hanlin, R.T.; Rodriguez, C.; Archibald, D.D.; Rigsby, L.L.; Eriksson, K.L. Identification and retting efficiencies of fungi isolated from dew-retted flax in the United States and europe. Appl. Environ. Microbiol. 1997, 63, 3950–3956. [Google Scholar]
- Mazian, B.; Bergeret, A.; Benezet, J.-C.; Malhautier, L. Influence of field retting duration on the biochemical, microstructural, thermal and mechanical properties of hemp fibres harvested at the beginning of flowering. Ind. Crops Prod. 2018, 116, 170–181. [Google Scholar] [CrossRef]
- Charlet, K.; Baley, C.; Morvan, C.; Jernot, J.P.; Gomina, M.; Bréard, J. Characteristics of Hermès flax fibres as a function of their location in the stem and properties of the derived unidirectional composites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1912–1921. [Google Scholar] [CrossRef]
- Liu, M.; Fernando, D.; Meyer, A.S.; Madsen, B.; Daniel, G.; Thygesen, A. Characterization and biological depectinization of hemp fibers originating from different stem sections. Ind. Crops Prod. 2015, 76, 880–891. [Google Scholar] [CrossRef]
- Hörtensteiner, S. The loss of green color during chlorophyll degradation—A prerequisite to prevent cell death? Planta 2004, 219, 191–194. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A. Why and what for the leaves are yellow in autumn? On the interpretation of optical spectra of senescing leaves (Acerplatanoides L.). J. Plant Physiol. 1995, 145, 315–320. [Google Scholar] [CrossRef]
- Matile, P. Biochemistry of indian summer: Physiology of autumnal leaf coloration. Exp. Gerontol. 2000, 35, 145–158. [Google Scholar] [CrossRef]
- Pallesen, B.E. The quality of combine-harvested fibre flax for industrials purposes depends on the degree of retting. Ind. Crops Prod. 1996, 5, 65–78. [Google Scholar] [CrossRef]
- Ribeiro, A.; Pochart, P.; Day, A.; Mennuni, S.; Bono, P.; Baret, J.; Spadoni, J.; Mangin, I. Microbial diversity observed during hemp retting. Appl. Microbiol. Biotechnol. 2015, 99, 4471–4484. [Google Scholar] [CrossRef]
- Liu, M.; Ale, M.T.; Kołaczkowski, B.; Fernando, D.; Daniel, G.; Meyer, A.S.; Thygesen, A. Comparison of traditional field retting and Phlebia radiata Cel 26 retting of hemp fibres for fibre-reinforced composites. AMB Express 2017, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Bleuze, L.; Lashermes, G.; Alavoine, G.; Recous, S.; Chabbert, B. Tracking the dynamics of hemp dew retting under controlled environmental conditions. Ind. Crops Prod. 2018, 123, 55–63. [Google Scholar] [CrossRef]
- Nykter, M.; Kyma, H.; Belinda, A.; Lilholt, H.; Thygesen, A. Effects of thermal and enzymatic treatments and harvesting time on the microbial quality and chemical composition of fibre hemp (Cannabis sativa L.). Biomass Bioenergy. 2008, 32, 392–399. [Google Scholar] [CrossRef]
- Meijer, W.J.M.; Vertregt, N.; Rutgers, B.; van de Waart, M. The pectin content as a measure of the retting and rettability of flax. Ind. Crops Prod. 1995, 4, 273–284. [Google Scholar] [CrossRef]
- Musialak, M.; Wróbel-Kwiatkowska, M.; Kulma, A.; Starzycka, E.; Szopa, J. Improving retting of fibre through genetic modification of flax to express pectinases. Transgenic Res. 2008, 17, 133–147. [Google Scholar] [CrossRef]
- Foulk, J.A.; Akin, D.E.; Dodd, R.B. Processing techniques for improving enzyme-retting of flax. Ind. Crops Prod. 2001, 13, 239–248. [Google Scholar] [CrossRef]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibres. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Li, Y.; Pickering, K.L.; Farrell, R.L. Determination of interfacial shear strength of white rot fungi treated hemp fibre reinforced polypropylene. Compos. Sci. Technol. 2009, 69, 1165–1171. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Baillie, C.A.; Matthews, F.L. Study of transcrystallinity and its effect on the interface in flax fibre reinforced composite materials. Compos. Part A Appl. Sci. Manuf. 2001, 32, 525–543. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Effect of various chemical treatments on the fibre structure and tensile properties of industrial hemp fibres. Compos. Part A Appl. Sci. Manuf. 2011, 42, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Bledzki, A.; Gassan, J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Placet, V.; Trivaudey, F.; Cisse, O.; Gucheret-Retel, V.; Boubakar, M.L. Diameter dependence of the apparent tensile modulus of hemp fibres: A morphological, structural or ultrastructural effect? Compos. Part A Appl. Sci. Manuf. 2012, 43, 275–287. [Google Scholar] [CrossRef]
- Ghetti, P.; Ricca, L.; Angelini, L. Thermal analysis of biomass and corresponding pyrolysis products. Fuel 1996, 75, 565–573. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Effects of chemical treatments on hemp fibre structure. Appl. Surf. Sci. 2013, 276, 13–23. [Google Scholar] [CrossRef]
- Manikandan Nair, K.C.; Thomas, S.; Groeninckx, G. Thermal and dynamic mechanical analysis of polystyrene composites reinforced with short sisal fibres. Compos. Sci. Technol. 2001, 61, 2519–2529. [Google Scholar] [CrossRef]
- Tanja Schäfer, B.H. Effect of sowing date and plant density on the cell morphology of hemp (Cannabis sativa L.). Ind. Crops Prod. 2006, 23, 88–98. [Google Scholar]
- Réquilé, S.; Goudenhooft, C.; Bourmaud, A.; Le Duigou, A.; Baley, C. Exploring the link between flexural behaviour of hemp and flax stems and fibre stiffness. Ind. Crops Prod. 2018, 113, 179–186. [Google Scholar] [CrossRef]
- Bourmaud, A.; Morvan, C.; Bouali, A.; Placet, V.; Perré, P.; Baley, C. Relationships between micro-fibrillar angle, mechanical properties and biochemical composition of flax fibers. Ind. Crops Prod. 2013, 44, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Placet, V.; François, C.; Day, A.; Beaugrand, J.; Ouagne, P. Industrial hemp transformation for composite applications: Influence of processing parameters on the fibre properties. Adv. Nat. Fibre Compos. 2018, 13–25. [Google Scholar]

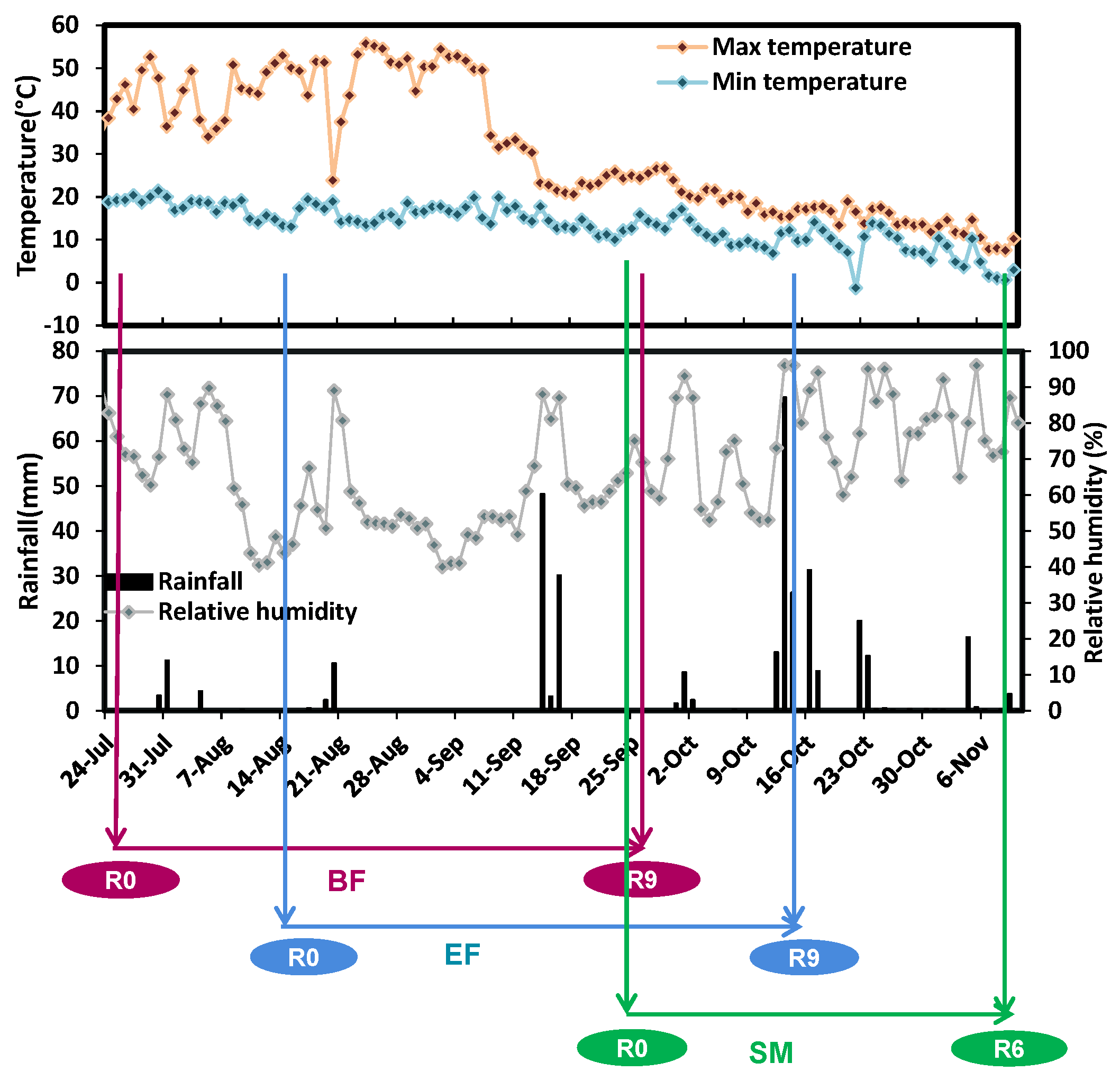


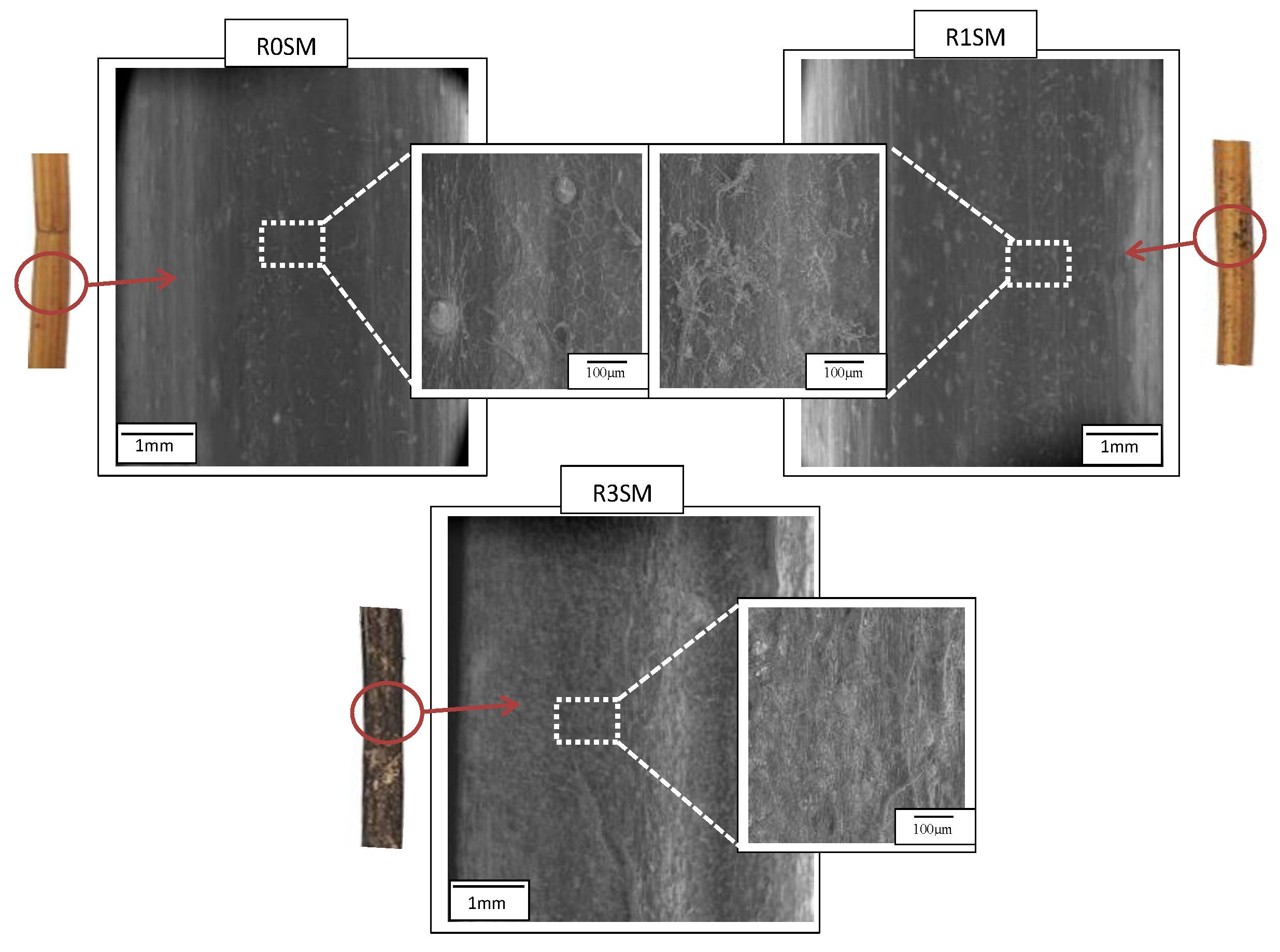


| Retting Time (Week) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Samples name | R0BF | R1BF | R2BF | R3BF | R4BF | R5BF | - | - | R9BF |
| R0EF | R1EF | R2EF | R3EF | - | R5EF | - | R7EF | R9EF | |
| R0SM | R1SM | R2SM | R3SM | R4SM | R5SM | R6SM | - | - |
| Crystallinity Order Index (%) | ||
|---|---|---|
| Retting Time | EF | SM |
| R0 | 58 | 64 |
| R3 | 67 | 71 |
| R5 | 68 | 73 |
| R9 | 69 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazian, B.; Bergeret, A.; Benezet, J.-C.; Malhautier, L. A Comparative Study of the Effect of Field Retting Time on the Properties of Hemp Fibres Harvested at Different Growth Stages. Fibers 2019, 7, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7120108
Mazian B, Bergeret A, Benezet J-C, Malhautier L. A Comparative Study of the Effect of Field Retting Time on the Properties of Hemp Fibres Harvested at Different Growth Stages. Fibers. 2019; 7(12):108. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7120108
Chicago/Turabian StyleMazian, Brahim, Anne Bergeret, Jean-Charles Benezet, and Luc Malhautier. 2019. "A Comparative Study of the Effect of Field Retting Time on the Properties of Hemp Fibres Harvested at Different Growth Stages" Fibers 7, no. 12: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7120108





