Liquid Phase Selective Hydrogenation of Phenol to Cyclohexanone over Electrospun Pd/PVDF-HFP Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pd/PVDF-HFP Catalyst
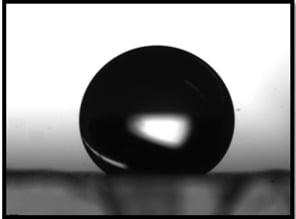
2.2. Catalytic Membrane Characterization
2.3. Catalytic Experiments
3. Results and Discussion
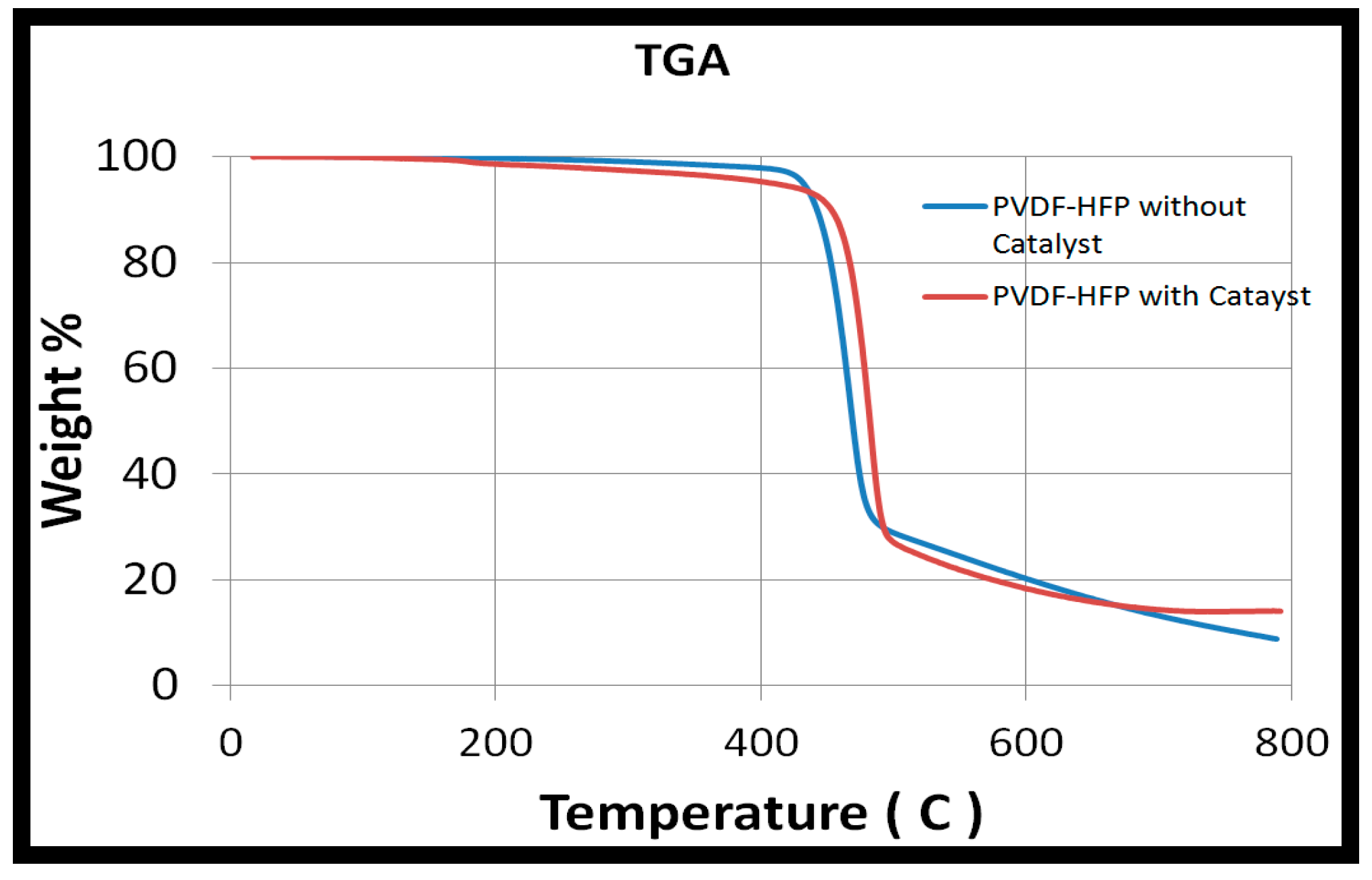
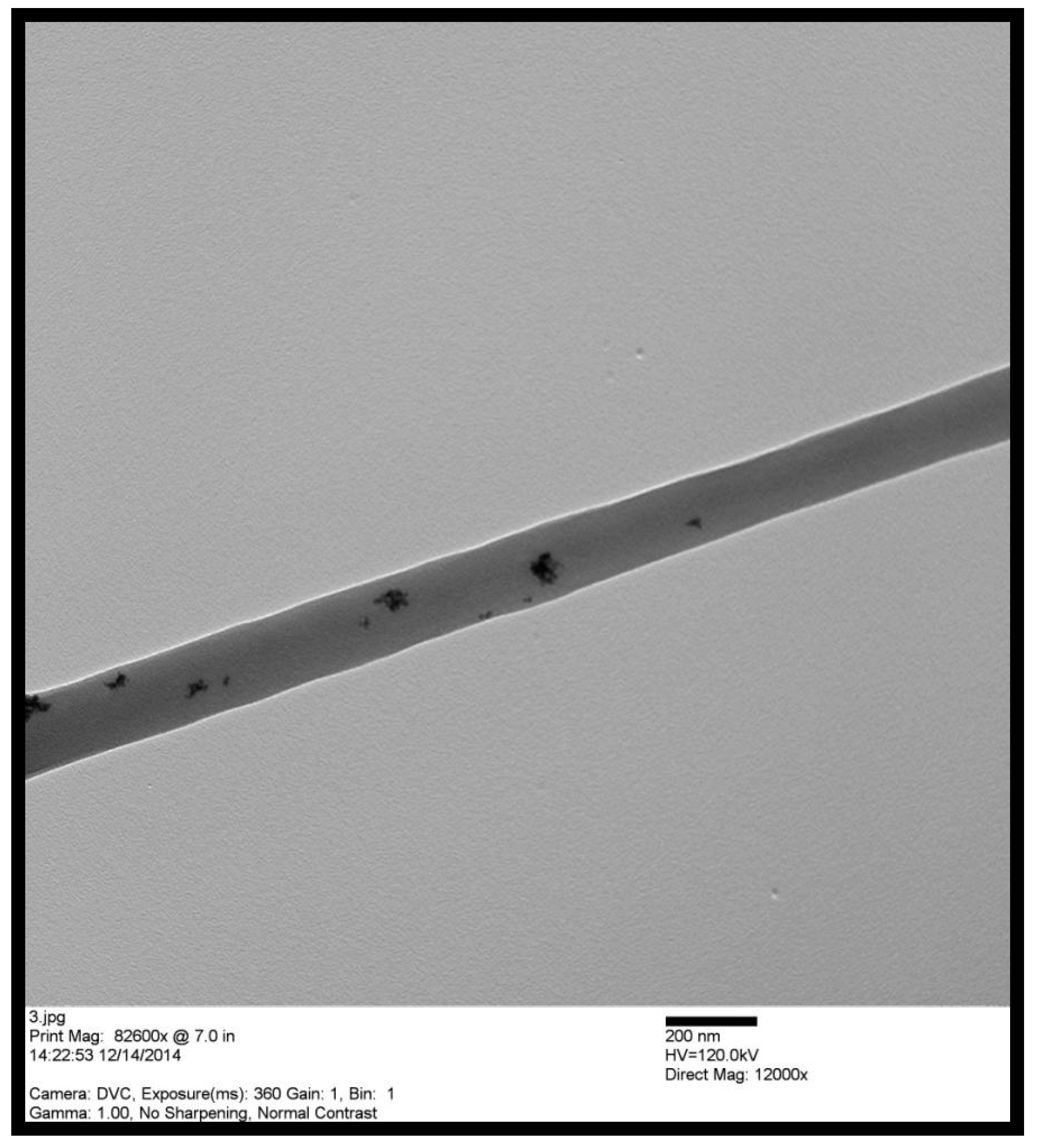
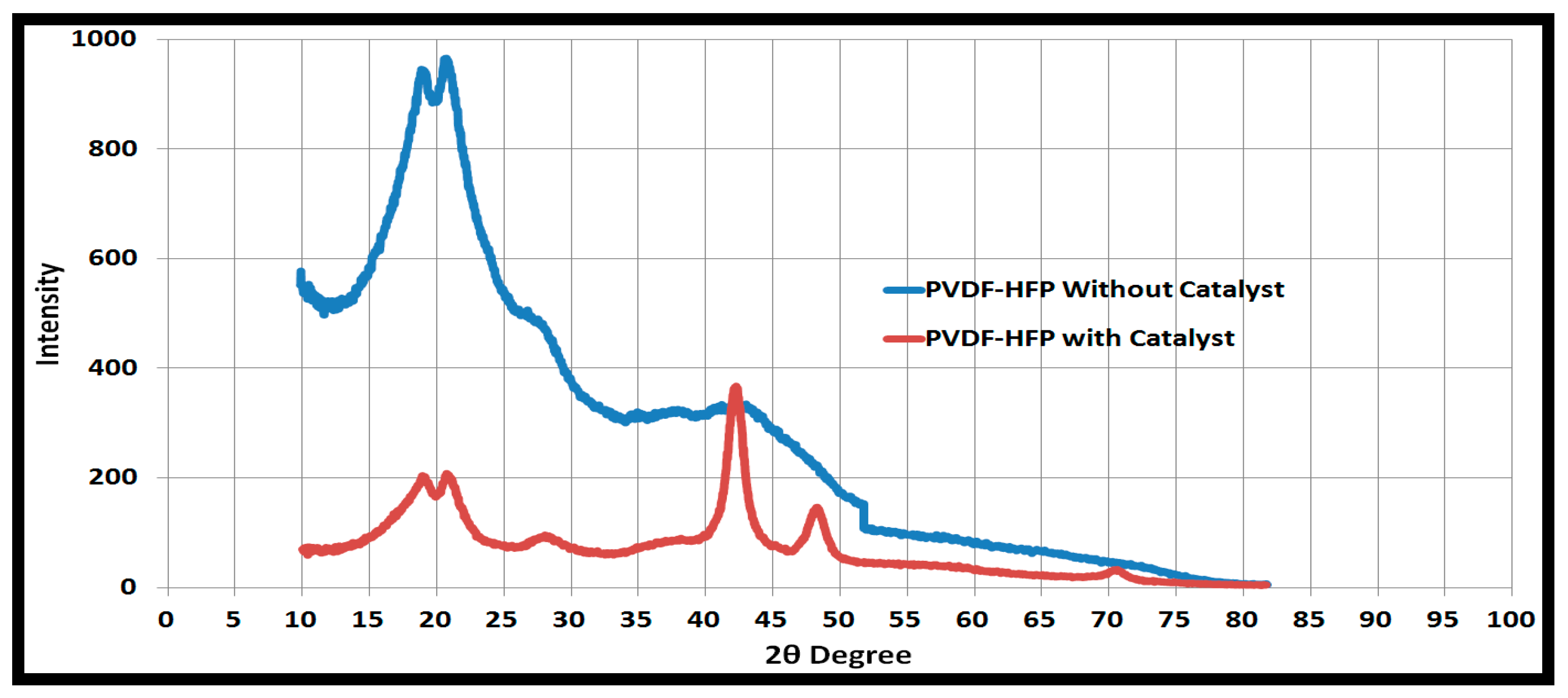
3.1. Pd/PVDF-HFP Characterization
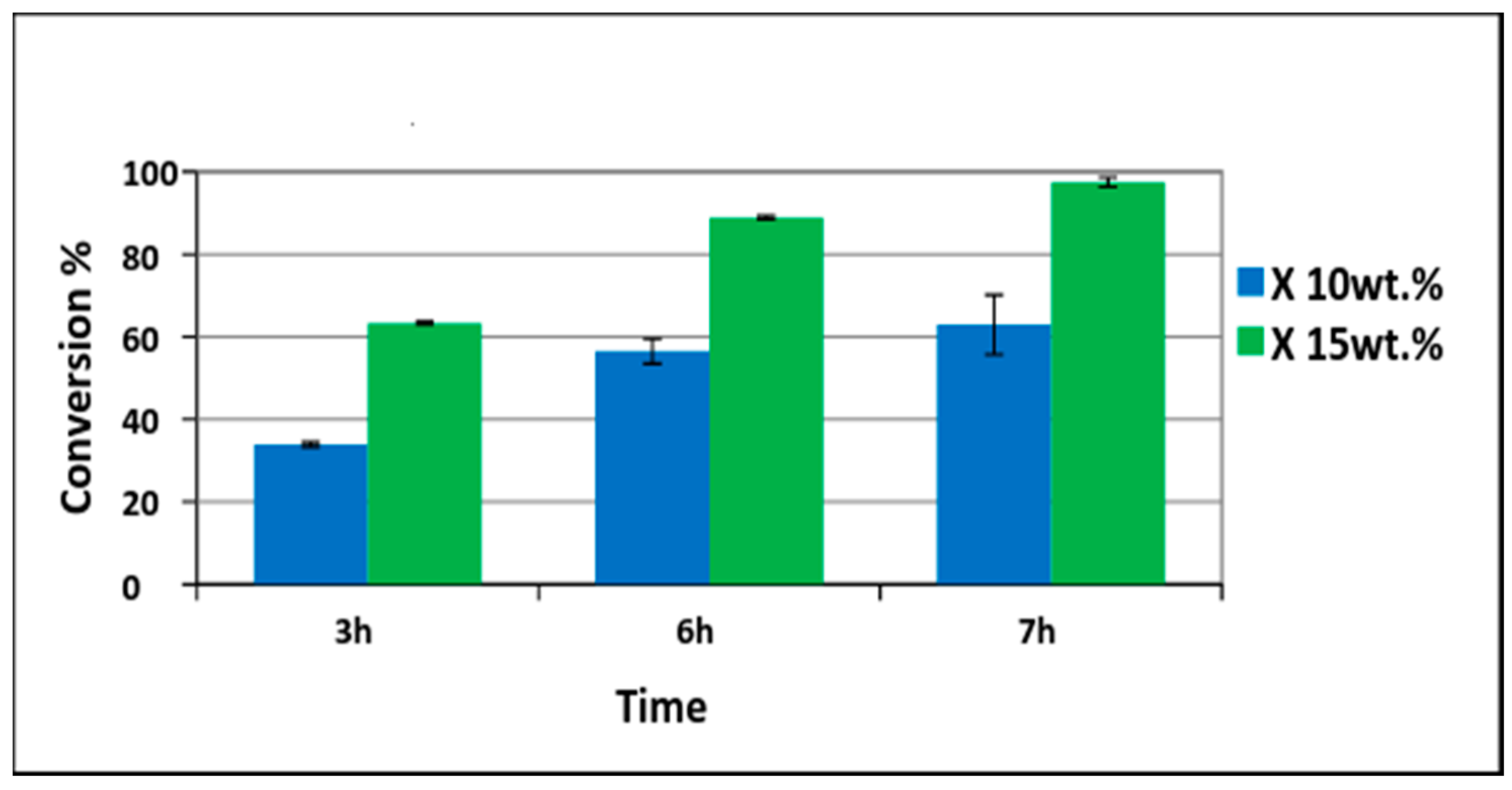
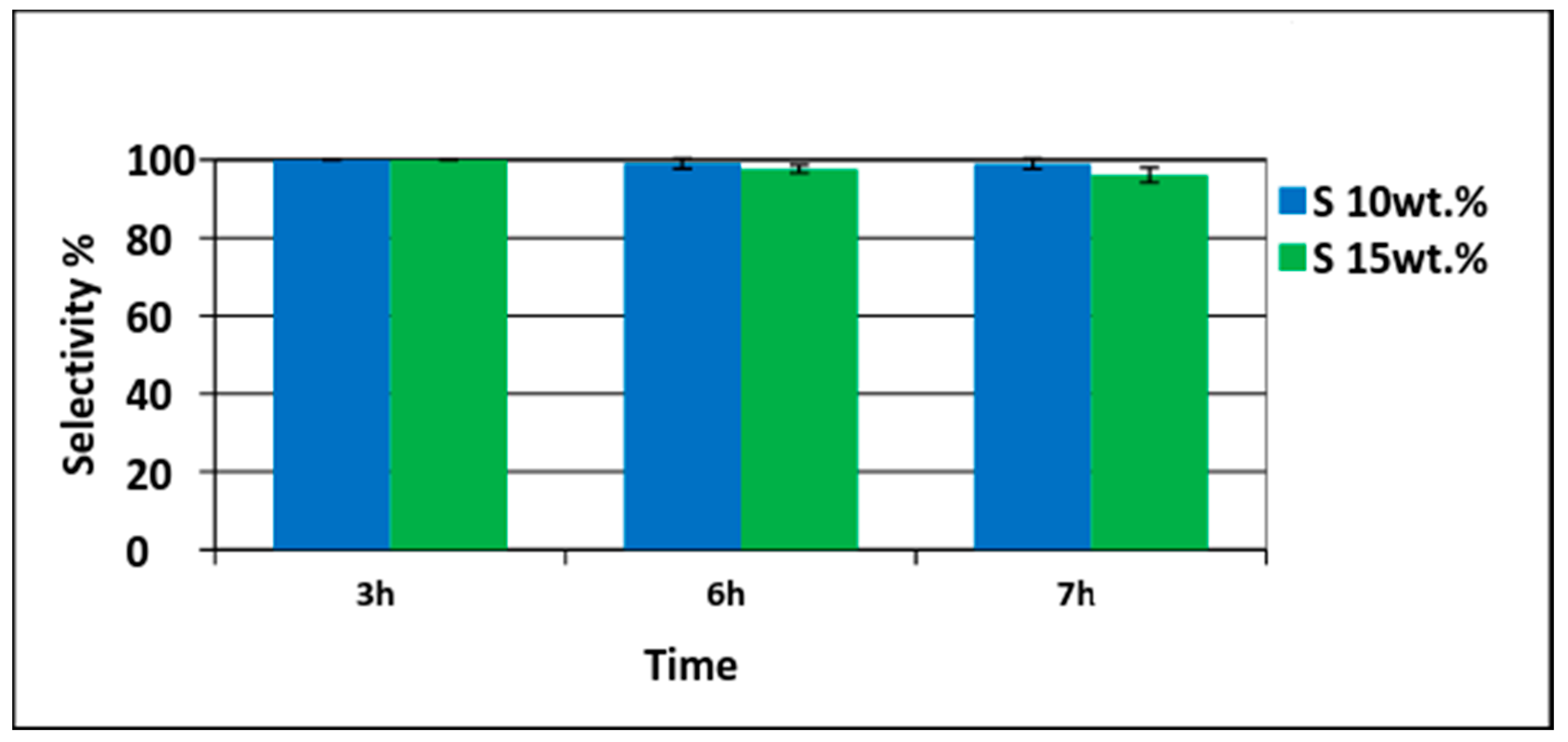
3.2. Pd/PVDF-HFP Catalytic Performance Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Jiang, T.; Han, B.; Liang, S.; Zhou, Y. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst. Science 2009, 326, 1250–1252. [Google Scholar] [CrossRef]
- Xiang, Y.; Ma, L.; Lu, C.; Zhang, Q.; Li, X. Aqueous system for the improved hydrogenation of phenol and its derivatives. Green Chem. 2008, 10, 939–943. [Google Scholar] [CrossRef]
- Fujita, S.; Yamada, T.; Akiyama, Y.; Cheng, H.; Arai, M. Hydrogenation of phenol with supported Rh catalysts in the presence of compressed CO2: Its effects on reaction rate, product selectivity and catalyst life. J. Supercrit. Fluids 2010, 54, 190–201. [Google Scholar] [CrossRef]
- Hu, S.; Yang, G.; Jiang, H.; Liu, Y.; Chen, R. Selective hydrogenation of phenol to cyclohexanone over Pd@CN (N-doped porous carbon): Role of catalyst reduction method. Appl. Surf. Sci. 2018, 435, 649–655. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, R.; Wang, Q.; Wu, C.; Yu, Y.; Zhao, F. Selective reduction of phenol derivatives to cyclohexanones in water under microwave irradiation. New J. Chem. 2012, 36, 1085–1090. [Google Scholar] [CrossRef]
- Yang, X.; Du, L.; Liao, S.; Li, Y.; Song, H. High-performance gold-promoted palladium catalyst towards the hydrogenation of phenol with mesoporous hollow spheres as support. Catal. Commun. 2012, 17, 29–33. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. N2O-Free single-pot conversion of cyclohexane to adipic acid catalysed by an iron(II) scorpionate complex. Green Chem. 2017, 19, 1499–1501. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1-yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.R.; Vishwanathan, V. Nanotubular titanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A Gen. 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, C.; Liu, Y.; Jiang, H.; Chen, R. Selective hydrogenation of phenol to cyclohexanone in water over Pd@N-doped carbons derived from ZIF-67: Role of dicyandiamide. Appl. Surf. Sci. 2017, 425, 484–491. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Long, L.; Wang, T.; Ma, L.; Wu, L.; Bai, Y.; Li, X.; Liao, S. Pt nanoparticles entrapped in titanate nanotubes (TNT) for phenol hydrogenation: the confinement effect of TNT. Chem. Commun. 2014, 50, 2794–2796. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Li, H. Liquid-phase selective hydrogenation of phenol to cyclohexanone over the Ce-doped Pd–B amorphous alloy catalyst. Mater. Lett. 2008, 62, 297–300. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, F.; Fujita, S.; Arai, M. Hydrogenation of phenol in scCO2 over carbon nanofiber supported Rh catalyst. Catal. Commun. 2008, 9, 362–368. [Google Scholar] [CrossRef]
- Chatterjee, M.; Kawanami, H.; Sato, M.; Chatterjee, A.; Yokoyama, T.; Suzuki, T. Hydrogenation of Phenol in Supercritical Carbon Dioxide Catalyzed by Palladium Supported on Al-MCM-41: A Facile Route for One-Pot Cyclohexanone Formation. Adv. Synth. Catal. 2009, 351, 1912–1924. [Google Scholar] [CrossRef]
- Velu, S.; Kapoor, M.P.; Inagaki, S.; Suzuki, K. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2. Appl. Catal. A Gen. 2003, 245, 317–331. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Q.; Cen, J.; Xiang, Y.; Li, X. Selectivity tailoring of Pd/CNTs in phenol hydrogenation by surface modification: Role of CO oxygen species. Appl. Surface Sci. 2015, 324, 634–639. [Google Scholar] [CrossRef]
- Makowski, P.; Cakan, R.D.; Antonietti, M.; Goettmann, F.; Titirici, M.-M. Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chem. Commun. 2008, 8, 999–1001. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.U.; Lolla, D.; Nikolov, Z.; Chase, G.G. Pd–Au nanoparticles supported by TiO2 fibers for catalytic NO decomposition by CO. J. Ind. Eng. Chem. 2016, 33, 91–98. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Li, H. Liquid-Phase Selective Hydrogenation of Phenol to Cyclohexanone over Pd-Ce-B/Hydrotalcite Catalyst. Chin. J. Catal. 2007, 28, 312–316. [Google Scholar] [CrossRef]
- Mahata, N.; Vishwanathan, V. Influence of Palladium Precursors on Structural Properties and Phenol Hydrogenation Characteristics of Supported Palladium Catalysts. J. Catal. 2002, 196, 262–270. [Google Scholar] [CrossRef]
- Sulman, E.M.; Ivanov, A.A.; Chernyavsky, V.S.; Sulman, M.G.; Bykov, A.I.; Sidorov, А.I.; Doluda, V.Y.; Matveeva, V.G.; Bronstein, L.M.; Stein, B.D. Kinetics of phenol hydrogenation over Pd-containing hypercrosslinked polystyrene. Chem. Eng. J. 2011, 33, 176–177. [Google Scholar] [CrossRef]
- Matos, J.; Corma, A. Selective phenol hydrogenation in aqueous phase on Pd-based catalysts supported on hybrid TiO2-carbon materials. Appl. Catal. A Gen. 2011, 404, 103–112. [Google Scholar] [CrossRef]
- Pérez, Y.; Fajardo, M.; Corma, A. Highly selective palladium supported catalyst for hydrogenation of phenol in aqueous phase. Catal. Commun. 2011, 12, 1071–1074. [Google Scholar] [CrossRef]
- Demir, M.M.; Gulgun, M.A.; Menceloglu, Y.Z.; Erman, B.; Yolu, R.; Abramchuk, S.S.; Makhaeva, E.E.; Matveeva, A.R.; Sulman, M.G. Palladium Nanoparticles by Electrospinning from Poly(acrylonitrile-co-acrylic acid)−PdCl2 Solutions. Relations between Preparation Conditions, Particle Size, and Catalytic Activity. Macromolecules 2004, 37, 1787–1792. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Wang, S.; Shen, M.; Guo, R.; Cao, X.; Zhu, M.; Shi, X. Efficient catalytic reduction of hexavalent chromium using palladium nanoparticle-immobilized electrospun polymer nanofibers. ACS Appl. Mater. Interfaces 2012, 4, 3054–3061. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. PVDF−HFP Membrane Formation by Supercritical CO2 Processing: Elucidation of Formation Mechanisms. Ind. Eng. Chem. Res. 2006, 45, 8939–8945. [Google Scholar] [CrossRef]
- Cardea, S.; Reverchon, E. Nanostructured PVDF-HFP membranes loaded with catalyst obtained by supercritical CO2 assisted techniques. Chem. Eng. Process Process Intensif. 2011, 50, 630–636. [Google Scholar] [CrossRef]
- Priya, A.R.S.; Subramania, A.; Jung, Y.; Kim, K. High-performance quasi-solid-state dye-sensitized solar cell based on an electrospun PVdF−HFP membrane electrolyte. Langmuir 2008, 24, 9816–9819. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Huang, J.; Xu, J.J.; Khalfan, A.; Greenbaum, S.G. Li Ion Conducting Polymer Gel Electrolytes Based on Ionic Liquid/PVDF-HFP Blends. J. Electrochem. Soc. 2007, 154, A1048–A1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.U.; Patel, S.U.; Chase, G.G. Electrospun superhydrophobic poly(vinylidene fluoride-co-hexafluoropropylene) fibrous membranes for the separation of dispersed water from ultralow sulfur diesel. Energy Fuels 2013, 27, 2458–2464. [Google Scholar] [CrossRef]
- Carlin, R.T.; Fuller, J. Ionic liquid–polymer gel catalytic membrane. Chem. Commun. 1997, 1345–1346. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Frenot, A.; Chronakis, I.S. Polymer Nanofibers Assembled by Electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Park, S.J. Catalytic Decomposition of Nitric Oxide and Carbon Monoxide Gases Using Nanofiber Based Filter Media. Ph.D. Dissertation, The University of Akron, Akron, OH, USA, August 2008. [Google Scholar]
- Fan, L.; Zhang, L.; Shen, Y.; Liu, D.; Wahab, N.; Hasan, M.M. Liquid-phase Hydrogenation of Phenol to Cyclohexanone over Supported Palladium Catalysts. Bull. Chem. Reac. Eng. Catal. 2016, 11, 354–360. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Chen, J.; Zhao, G.; Ma, L.; Yu, Y. Selective Hydrogenation of Phenol and Derivatives over Polymer-Functionalized Carbon-Nanofiber-Supported Palladium Using Sodium Formate as the Hydrogen Source. ChemPlusChem 2013, 78, 1370–1378. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning Over” Definitions in Catalytic Cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abutaleb, A.; Lolla, D.; Aljuhani, A.; Shin, H.U.; Ali, M.A.; Yousef Hassan, A.A.; Maafa, I.M.H.; Chase, G.G. Liquid Phase Selective Hydrogenation of Phenol to Cyclohexanone over Electrospun Pd/PVDF-HFP Catalyst. Fibers 2019, 7, 28. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7040028
Abutaleb A, Lolla D, Aljuhani A, Shin HU, Ali MA, Yousef Hassan AA, Maafa IMH, Chase GG. Liquid Phase Selective Hydrogenation of Phenol to Cyclohexanone over Electrospun Pd/PVDF-HFP Catalyst. Fibers. 2019; 7(4):28. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7040028
Chicago/Turabian StyleAbutaleb, Ahmed, Dinesh Lolla, Abdulwahab Aljuhani, Hyeon Ung Shin, Mohammad Ashraf Ali, Aymen Ahmed Yousef Hassan, Ibrahim Mohammed Hassan Maafa, and George G. Chase. 2019. "Liquid Phase Selective Hydrogenation of Phenol to Cyclohexanone over Electrospun Pd/PVDF-HFP Catalyst" Fibers 7, no. 4: 28. https://0-doi-org.brum.beds.ac.uk/10.3390/fib7040028