Mechanical and Structural Characterization of Pineapple Leaf Fiber
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Methods
2.2.1. Extraction and Alkali Treatment of PALF
2.2.2. Fiber Tensile Test
2.3. Characterization of PALF
2.3.1. X-ray Diffraction (XRD) Analysis
2.3.2. FTIR Analysis
2.4. Statistical Data Analysis
3. Results
3.1. Fiber Tensile Test
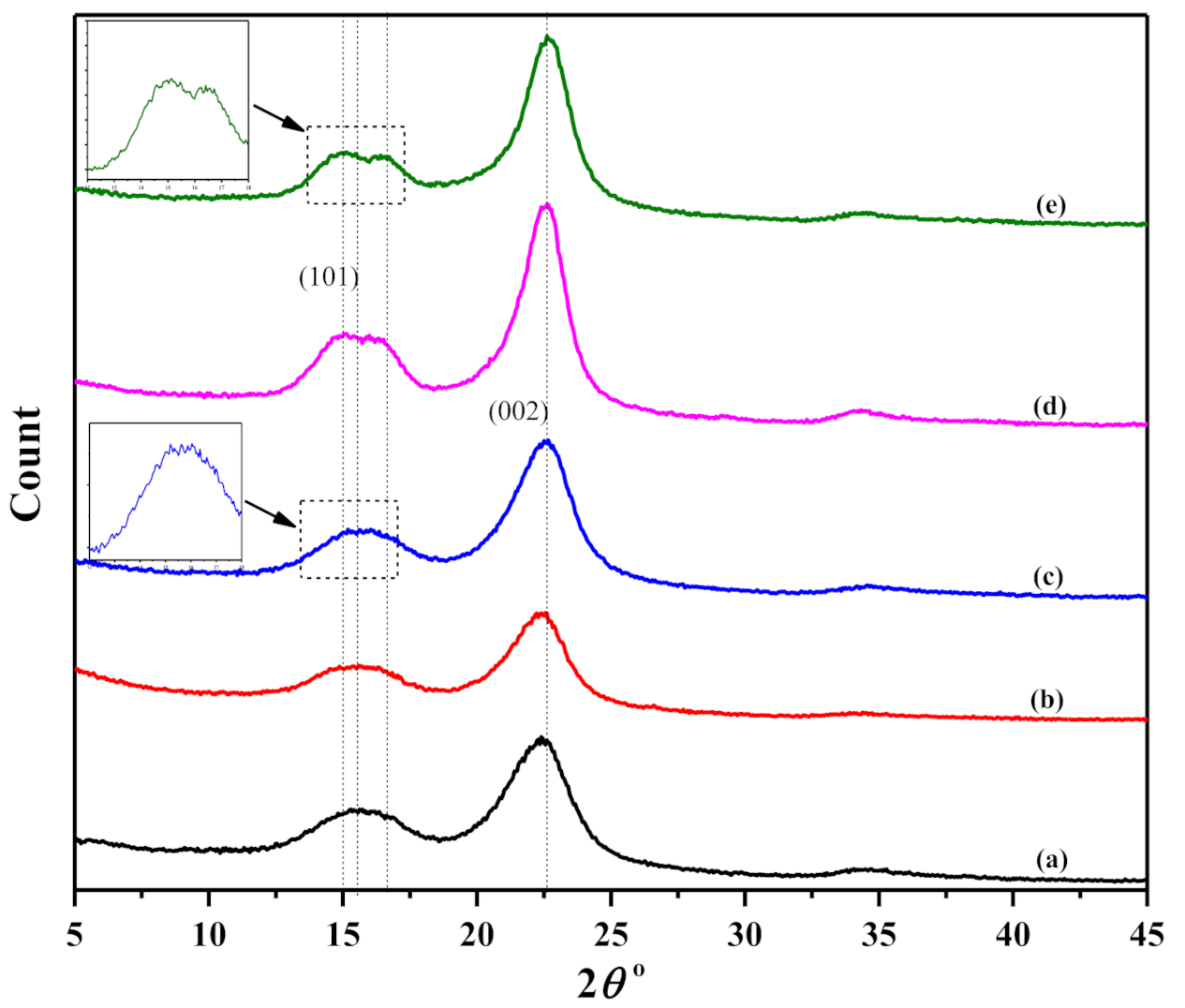
3.2. XRD Analysis: Crystallinity and Crystallite Size
3.3. FTIR Spectral Analysis
4. Discussion
4.1. The Influence of Crystal Properties on the Mechanical Behaviour of PALF
4.2. Effect of NaOH Treatment on the Crystalline Properties of PALF
4.3. Effect of NaOH Treatment on the Chemical Composition of PALF
4.4. Potential Polymer Reinforcement Using PALF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cislaghi, A.; Sala, P.; Borgonovo, G.; Gandolfi, C.; Bischetti, G. Towards More Sustainable Materials for Geo-Environmental Engineering: The Case of Geogrids. Sustainability 2021, 13, 2585. [Google Scholar] [CrossRef]
- Diabor, E.; Funkenbusch, P.; Kaufmann, E.E. Characterization of Cassava Fiber of Different Genotypes as a Potential Reinforcement Biomaterial for Possible Tissue Engineering Composite Scaffold Application. Fibers Polym. 2019, 20, 217–228. [Google Scholar] [CrossRef]
- Essel, T.Y.A.; Koomson, A.; Seniagya, M.-P.O.; Cobbold, G.P.; Kwofie, S.K.; Asimeng, B.O.; Arthur, P.K.; Awandare, G.; Tiburu, E.K. Chitosan Composites Synthesized Using Acetic Acid and Tetraethylorthosilicate Respond Differently to Methylene Blue Adsorption. Polymers 2018, 10, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odusote, J.K.; Oyewo, A.T. Mechanical Properties of Pineapple Leaf Fiber Reinforced Polymer Composites for Application as a Prosthetic Socket. J. Eng. Technol. 2016, 7, 125–139. [Google Scholar]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Jawaid, M.; Asim, M.; Tahir, P.M.; Nasir, M. (Eds.) Pineapple Leaf Fibers: Processing, Properties and Applications; Green Energy and Technology; Springer: Singapore, 2020; ISBN 9789811514159. [Google Scholar]
- Todkar, S.S.; Patil, S.A. Review on mechanical properties evaluation of pineapple leaf fibre (PALF) reinforced polymer composites. Compos. Part B Eng. 2019, 174, 106927. [Google Scholar] [CrossRef]
- Glória, G.O.; Teles, M.C.A.; Lopes, F.P.D.; Vieira, C.M.F.; Margem, F.M.; Gomes, M.D.A.; Monteiro, S.N. Tensile strength of polyester composites reinforced with PALF. J. Mater. Res. Technol. 2017, 6, 401–405. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E. A Review on Pineapple Leaves Fibre and Its Composites. Int. J. Polym. Sci. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Salim, R.; Ammayappan, L. An Overview on Production, Properties, and Value Addition of Pineapple Leaf Fibers (PALF). J. Nat. Fibers 2016, 13, 362–373. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefin. 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Abdan, K.; Nasir, M. Effect of Alkali treatments on physical and Mechanical strength of Pineapple leaf fibres. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 12030. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Siengchin, S.; Parameswaranpillai, J.; Jawaid, M.; Pruncu, C.I.; Khan, A. A comprehensive review of techniques for natural fibers as reinforcement in composites: Preparation, processing and characterization. Carbohydr. Polym. 2019, 207, 108–121. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Alpar, T. Potential Natural Fiber Polymeric Nanobiocomposites: A Review. Polymers 2020, 12, 1072. [Google Scholar] [CrossRef]
- Hoque, M.B.; Solaiman; Alam, A.H.; Mahmud, H.; Nobi, A. Mechanical, Degradation and Water Uptake Properties of Fabric Reinforced Polypropylene Based Composites: Effect of Alkali on Composites. Fibers 2018, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of Nanocellulose from Pineapple Leaf Fibers via High-Shear Homogenization and Ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Herrera-Franco, P.; Valadez-González, A. A study of the mechanical properties of short natural-fiber reinforced composites. Compos. Part B Eng. 2005, 36, 597–608. [Google Scholar] [CrossRef]
- C28 Committee Test Method for Tensile Strength and Youngs Modulus of Fibers; ASTM International: West Conshohocken, PA, USA, 2014.
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S. All-cellulose composite laminates prepared from pineapple leaf fibers treated with steam explosion and alkaline treatment. J. Reinf. Plast. Compos. 2017, 36, 1146–1155. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Khalid, M.; Ratnam, C.T.; Manickam, S.; Walvekar, R.; Hoque, E. Sonosynthesis of cellulose nanoparticles (CNP) from kenaf fiber: Effects of processing parameters. Fibers Polym. 2016, 17, 1352–1358. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xue, S.; Zhang, P.; Tian, Y.; Li, P. Application of Natural Plant Fibers in Cement-Based Composites and the Influence on Mechanical Properties and Mass Transport. Materials 2019, 12, 3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petroudy, S.D. Physical and mechanical properties of natural fibers. In Advanced High Strength Natural Fibre Composites in Construction; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 59–83. [Google Scholar]
- Komuraiah, A.; Kumar, N.S.; Prasad, B.D. Chemical Composition of Natural Fibers and its Influence on their Mechanical Properties. Mech. Compos. Mater. 2014, 50, 359–376. [Google Scholar] [CrossRef]
- Neto, A.R.S.; Araujo, M.A.; Barboza, R.M.; Fonseca, A.S.; Tonoli, G.; Souza, F.; Mattoso, L.H.; Marconcini, J.M. Comparative study of 12 pineapple leaf fiber varieties for use as mechanical reinforcement in polymer composites. Ind. Crops Prod. 2015, 64, 68–78. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Jain, J.; Sinha, S.; Jain, S. Compendious Characterization of Chemically Treated Natural Fiber from Pineapple Leaves for Reinforcement in Polymer Composites. J. Nat. Fibers 2021, 18, 845–856. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, A.; Fan, S.; Liu, Y.; Liu, X. Crystal Structure and Mechanical Properties of Uniaxially Stretched PA612/SiO2 Films. Polymers 2020, 12, 711. [Google Scholar] [CrossRef] [Green Version]
- Zin, M.H.; Abdan, K.; Mazlan, N.; Zainudin, E.S.; E Liew, K. The effects of alkali treatment on the mechanical and chemical properties of pineapple leaf fibres (PALF) and adhesion to epoxy resin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 012035. [Google Scholar] [CrossRef]
- Duval, A.; Bourmaud, A.; Augier, L.; Baley, C. Influence of the sampling area of the stem on the mechanical properties of hemp fibers. Mater. Lett. 2011, 65, 797–800. [Google Scholar] [CrossRef]
- Das, M.; Pal, A.; Chakraborty, D. Effects of mercerization of bamboo strips on mechanical properties of unidirectional bamboo–novolac composites. J. Appl. Polym. Sci. 2006, 100, 238–244. [Google Scholar] [CrossRef]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Effect of Alkali Treatment on Crystalline Structure of Cellulose Fiber from Mendong (Fimbristylis globulosa) Straw. Key Eng. Mater. 2013, 594, 720–724. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef] [Green Version]
- Cui, Z.; Shi, J.; Wan, C.; Li, Y. Comparison of alkaline- and fungi-assisted wet-storage of corn stover. Bioresour. Technol. 2012, 109, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mago, G.; Balan, V.; Wyman, C.E. Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies. Bioresour. Technol. 2009, 100, 3948–3962. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.F.; Dietrich, A.; Goring, D.A.I. Effect of mercerization on the crystallite size and crystallinity index in cellulose from different sources. Can. J. Chem. 1987, 65, 1724–1725. [Google Scholar] [CrossRef]
- Reddy, K.O.; Shukla, M.; Maheswari, C.U.; Rajulu, A.V. Mechanical and physical characterization of sodium hydroxide treated Borassus fruit fibers. J. For. Res. 2012, 23, 667–674. [Google Scholar] [CrossRef]
- Asim, M.; Jawaid, M.; Abdan, K.; Ishak, M.R. Effect of Alkali and Silane Treatments on Mechanical and Fibre-matrix Bond Strength of Kenaf and Pineapple Leaf Fibres. J. Bionic Eng. 2016, 13, 426–435. [Google Scholar] [CrossRef]
- Samal, R.K.; Ray, M.C. Effect of Chemical Modifications on FTIR Spectra. II. Physicochemical Behavior of Pineapple Leaf Fiber (PALF). J. Appl. Polym. Sci. 1997, 64, 2119–2125. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Shi, X.; Wang, L. Effect of Hot-Alkali Treatment on the Structure Composition of Jute Fabrics and Mechanical Properties of Laminated Composites. Materials 2019, 12, 1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Małachowska, E.; Dubowik, M.; Lipkiewicz, A.; Przybysz, K.; Przybysz, P. Analysis of Cellulose Pulp Characteristics and Processing Parameters for Efficient Paper Production. Sustainability 2020, 12, 7219. [Google Scholar] [CrossRef]

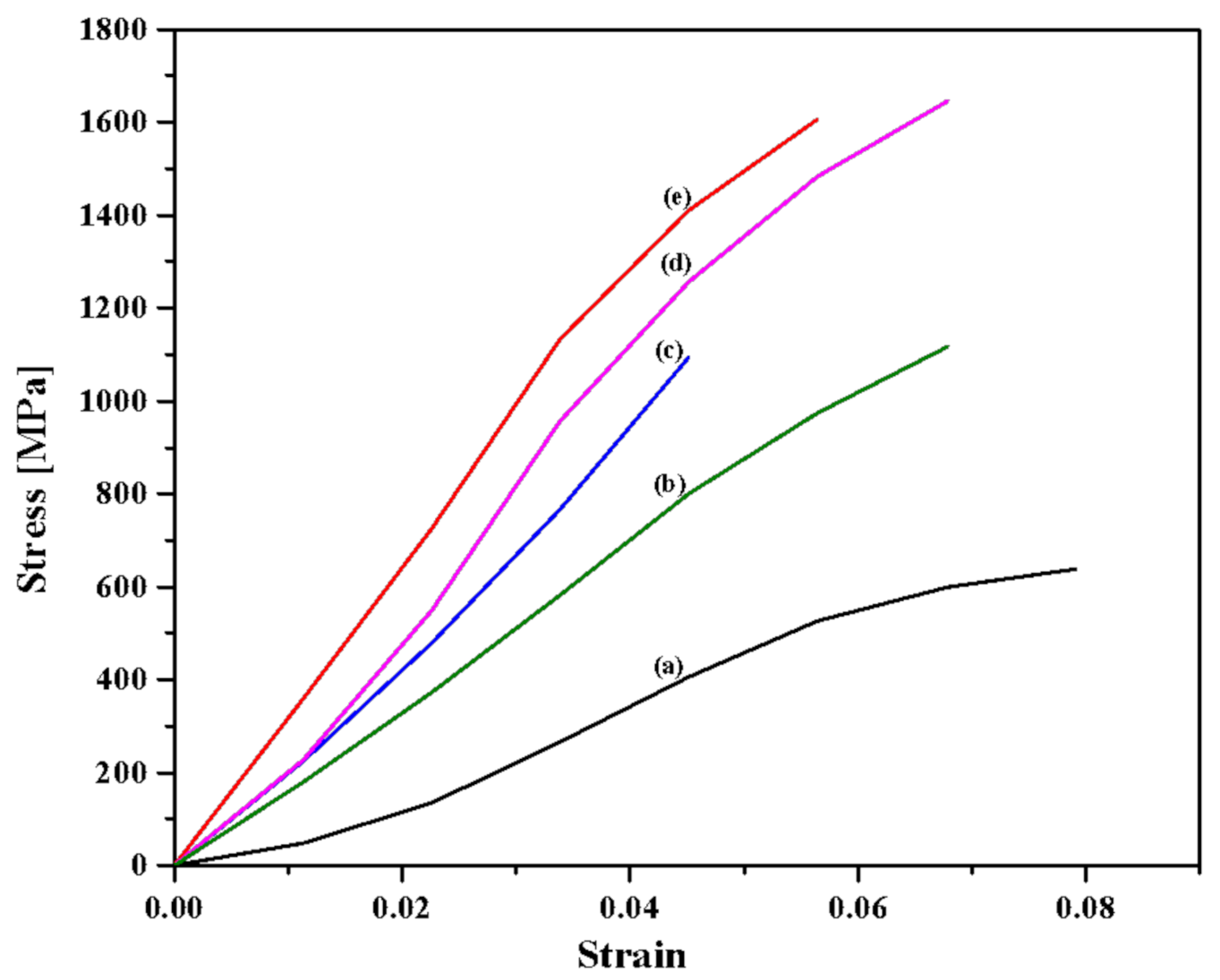

| PALF Samples | Ultimate Tensile Strength (MPa) | Young’s Modulus (GPa) | Strain at Break (%) |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Untreated PALF | 630 ± 50 * | 9 ± 1.1 * | 7.9 ± 0.7 *** |
| 1% NaOH | 1560 ± 270 *** | 29 ± 2.2 **** | 5.6 ± 0.1 ** |
| 3% NaOH | 1090 ± 166 ** | 24 ± 0.7 *** | 4.5 ± 0.1 * |
| 6% NaOH | 1620 ± 150 *** | 27 ± 0.9 **** | 6.7 ± 0.5 *** |
| 9% NaOH | 1100 ± 56 ** | 17 ± 0.2 ** | 6.7 ± 0.4 *** |
| PALF Sample | Crystallinity (%) | Crystallite Size (nm) |
|---|---|---|
| Untreated PALF | 62.2 | 18.53 |
| 1% NaOH | 65.9 | 21.39 |
| 3% NaOH | 67.4 | 20.00 |
| 6% NaOH | 75.6 | 24.00 |
| 9% NaOH | 71.6 | 23.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaba, E.W.; Asimeng, B.O.; Kaufmann, E.E.; Katu, S.K.; Foster, E.J.; Tiburu, E.K. Mechanical and Structural Characterization of Pineapple Leaf Fiber. Fibers 2021, 9, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/fib9080051
Gaba EW, Asimeng BO, Kaufmann EE, Katu SK, Foster EJ, Tiburu EK. Mechanical and Structural Characterization of Pineapple Leaf Fiber. Fibers. 2021; 9(8):51. https://0-doi-org.brum.beds.ac.uk/10.3390/fib9080051
Chicago/Turabian StyleGaba, Eric Worlawoe, Bernard O. Asimeng, Elsie Effah Kaufmann, Solomon Kingsley Katu, E. Johan Foster, and Elvis K. Tiburu. 2021. "Mechanical and Structural Characterization of Pineapple Leaf Fiber" Fibers 9, no. 8: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/fib9080051






