Biowaste-Derived Humic-like Substances Improve Growth and Quality of Orange Jasmine (Murraya paniculata L. Jacq.) Plants in Soilless Potted Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biowaste-Derived and Commercial Humic-like Substances
2.2. Tested Plant and Growing Conditions
2.3. Plant Growth Measurement
2.4. Leaf SPAD Index, Color Coordinates, Gas Exchanges Measurement and Total Chlorophyll Content
2.5. Experimental Design and Data Analysis
3. Results
3.1. Chemical Characteristics of Leonardite-Based and Biowaste-Derived HLS
3.2. Plant Growth, Quality Traits, Biomass Production and WUE
3.3. Leaf SPAD Index, Gas Exchanges, Total Chlorophyll Content and Color Coordinates
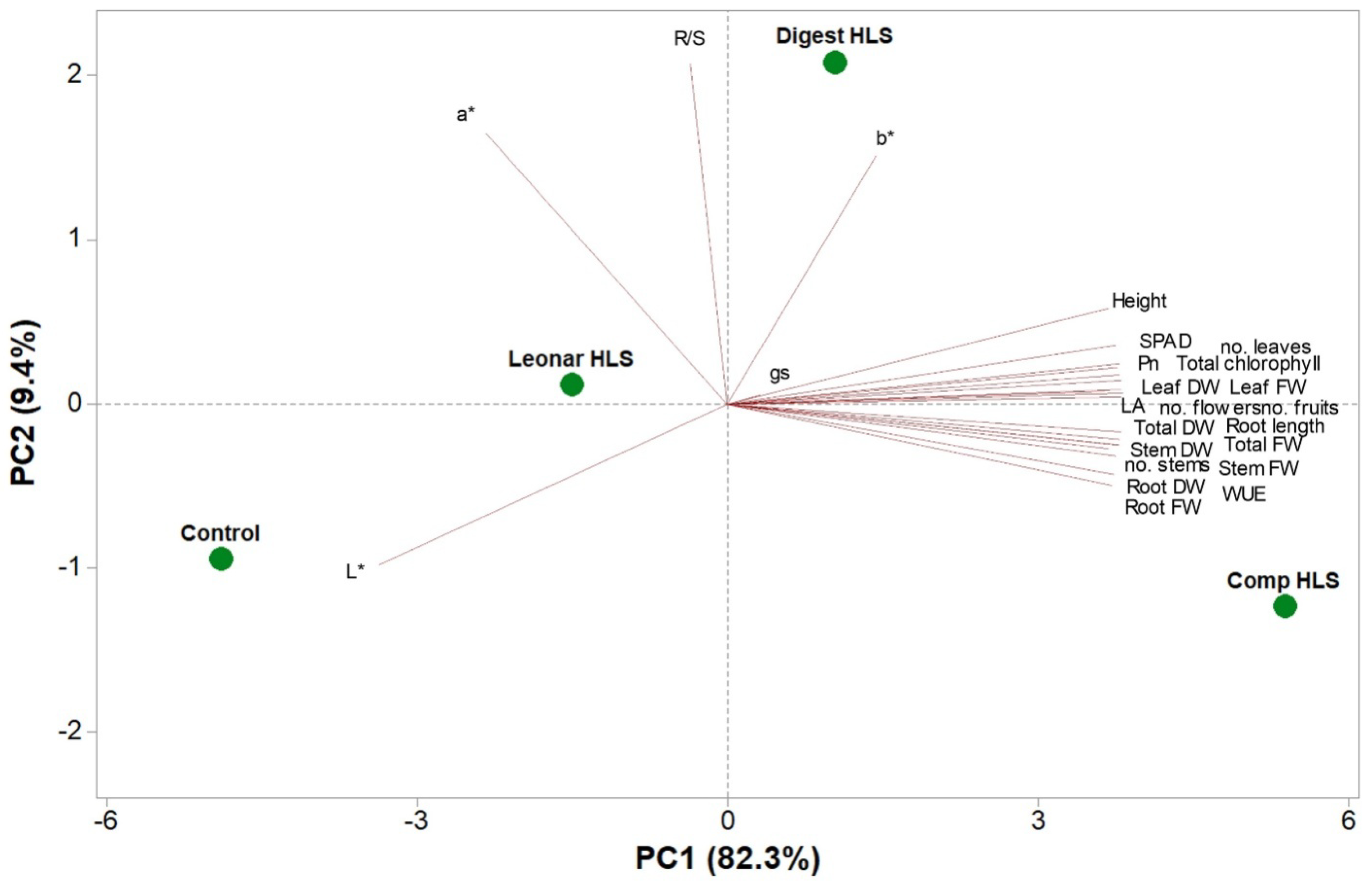
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Spíchal, L.; Panzarová, K.; Casa, R.; Colla, G. High-Throughput Plant Phenotyping for Developing Novel Biostimulants: From Lab to Field or From Field to Lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Guilayn, F.; Benbrahim, M.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Humic-like substances extracted from different digestates: First trials of lettuce biostimulation in hydroponic culture. Waste Manag. 2020, 104, 239–245. [Google Scholar] [CrossRef] [PubMed]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a Sustainable Agriculture Through Plant Biostimulants: From Experimental Data to Practical Applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Petropoulos, S.A. Practical Applications of Plant Biostimulants in Greenhouse Vegetable Crop Production. Agronomy 2020, 10, 1569. [Google Scholar] [CrossRef]
- de Pascale, S.; Rouphael, Y.; Cirillo, C.; Colla, G. Plant biostimulants in greenhouse horticulture: Recent advances and challenges ahead. Acta Hortic. 2020, 1271, 327–334. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Baglieri, A.; Cadili, V.; Tambone, F.; Gennari, M.; Nardi, S. Humic-like substances from agro-industrial residues affect growth and nitrogen assimilation in maize (Zea mays L.) plantlets. J. Geochem. Explor. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Fascella, G.; Montoneri, E.; Ginepro, M.; Francavilla, M. Effect of urban biowaste derived soluble substances on growth, photosynthesis and ornamental value of Euphorbia x lomi. Sci. Hortic. 2015, 197, 90–98. [Google Scholar] [CrossRef]
- Fascella, G.; Montoneri, E.; Francavilla, M. Biowaste versus fossil sourced auxiliaries for plant cultivation: The Lantana case study. J. Clean. Prod. 2018, 185, 322–330. [Google Scholar] [CrossRef]
- Pereira, M.M.A.; Morais, L.C.; Marques, E.A.; Martins, A.D.; Cavalcanti, V.P.; Rodrigues, F.A.; Gonçalves, W.M.; Blank, A.F.; Pasqual, M.; Dória, J. Humic Substances and Efficient Microorganisms: Elicitation of Medicinal Plants—A Review. J. Agric. Sci. 2019, 11, 268–280. [Google Scholar] [CrossRef]
- Montoneri, E. Municipal waste treatment, technological scale up and commercial exploitation: The case of bio-waste lignin to soluble lignin-like polymers. In Food Waste Reduction and Valorisation: Sustainability Assessment and Policy Analysis; Morone, P., Papendiek, F., Tartiu, V.E., Eds.; Springer: Cham, Switzerland, 2017; pp. 79–120. ISBN 9783319500881. [Google Scholar]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like bioactivity on emergence and early growth of maize (Zea mays L.) of water-soluble lignins isolated from biomass for energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Drosos, M.; Nebbioso, A.; Mazzei, P.; Vinci, G.; Spaccini, R.; Piccolo, A. A molecular zoom into soil Humeome by a direct sequential chemical fractionation of soil. Sci. Total. Environ. 2017, 586, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T. Humic supramolecular structures have polar surfaces and unpolar cores in native soil. Chemosphere 2017, 183, 437–443. [Google Scholar] [CrossRef]
- Nunes, R.D.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nogueira, F.C.S.; Canellas, L.P.; Olivares, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic Substances Contribute to Plant Iron Nutrition Acting as Chelators and Biostimulants. Front. Plant Sci. 2019, 10, 675. [Google Scholar] [CrossRef] [Green Version]
- Jindo, K.; Olivares, F.L.; Malcher, D.J.D.P.; Sánchez-Monedero, M.A.; Kempenaar, C.; Canellas, L.P. From Lab to Field: Role of Humic Substances Under Open-Field and Greenhouse Conditions as Biostimulant and Biocontrol Agent. Front. Plant Sci. 2020, 11, 426. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Francioso, O. Soil-root cross-talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2016, 180, 5–13. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Canellas, N.O.A.; Mazzei, P.; Piccolo, A. Humic acids increase the maize seedlings exudation yield. Chem. Biol. Technol. Agric. 2019, 6, 3. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Selvam, A.; Wong, J.W. Evaluation of humic substances during co-composting of food waste, sawdust and Chinese medicinal herbal residues. Bioresour. Technol. 2014, 168, 229–234. [Google Scholar] [CrossRef]
- Palumbo, G.; Schiavon, M.; Nardi, S.; Ertani, A.; Celano, G.; Colombo, C.M. Biostimulant Potential of Humic Acids Extracted From an Amendment Obtained via Combination of Olive Mill Wastewaters (OMW) and a Pre-treated Organic Material Derived From Municipal Solid Waste (MSW). Front. Plant Sci. 2018, 9, 1028. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.-T.; Wu, S.-B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total. Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- Fascella, G.; Rouphael, Y. Growth and water use efficiency of potted Murraya paniculata as affected by irrigation system and container size. Eur. J. Hortic. Sci. 2015, 80, 81–86. [Google Scholar] [CrossRef]
- Massa, D.; Prisa, D.; Montoneri, E.; Battaglini, D.; Ginepro, M.; Negre, M.; Burchi, G. Application of municipal biowaste derived products in Hibiscus cultivation: Effect on leaf gaseous exchange activity, and plant biomass accumulation and quality. Sci. Hortic. 2016, 205, 59–69. [Google Scholar] [CrossRef]
- Massa, D.; Lenzi, A.; Montoneri, E.; Ginepro, M.; Prisa, D.; Burchi, G. Plant response to biowaste soluble hydrolysates in hibiscus grown under limiting nutrient availability. J. Plant Nutr. 2017, 41, 396–409. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci. Total. Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Olawore, N.O.; Ogunwande, I.; Ekundayo, O.; Adeleke, K.A. Chemical composition of the leaf and fruit essential oils of Murraya paniculata (L.) Jack. (Syn.Murraya exotica Linn.). Flavour Fragr. J. 2004, 20, 54–56. [Google Scholar] [CrossRef]
- Fascella, G.; Mammano, M.; Rouphael, Y.; Cirillo, C. Agronomical and physiological responses of containerized ornamentals to salinity induced by major nutrients. Acta Hortic. 2017, 1170, 635–642. [Google Scholar] [CrossRef]
- Fascella, G.; Rouphael, Y.; Cirillo, C.; Pannico, A.; El-Nakhel, C.; De Pascale, S. Growth and quality response of potted ornamental shrubs under salt stress. Acta Hortic. 2020, 1296, 861–868. [Google Scholar] [CrossRef]
- Fascella, G.; Mammano, M.; D’Angiolillo, F.; Cacini, S.; Massa, D.; Rouphael, Y. Biochar as growing substrate component for potted Murraya paniculata. Acta Hortic. 2021, 1305, 227–232. [Google Scholar] [CrossRef]
- D’Angiolillo, F.; Mammano, M.M.; Fascella, G. Pigments, Polyphenols and Antioxidant Activity of Leaf Extracts from Four Wild Rose Species Grown in Sicily. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Unitelma Sapienza. La Bioeconomia Circolare Urbana: Politiche e Norme per la Transizione. Webinar at Bio-Economy Day, Rome, 27 May 2021. Available online: www.unitelmasapienza.it (accessed on 24 June 2021).
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Baglieri, A.; Cadili, V.; Monterumici, C.M.; Gennari, M.; Tabasso, S.; Montoneri, E.; Nardi, S.; Negre, M. Fertilization of bean plants with tomato plants hydrolysates. Effect on biomass production, chlorophyll content and N assimilation. Sci. Hortic. 2014, 176, 194–199. [Google Scholar] [CrossRef]
| pH | C | N | C/N | P2O5 | K | Ca | Mg | Fe | Na | Cu | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leonar HLS | 9.9 ± 0.2 | 29.8 a ± 0.5 | 0.22 ± 0.01 | 135.4 ± 2.0 | 0.52 b ± 0.02 | 4.89 ± 0.03 | 0.41 ± 0.04 | 0.39 ± 0.02 | 0.10 ± 0.01 | 3.10 ± 0.06 | 2.96 ± 0.05 | 2.50 ± 0.06 |
| Digest HLS | 6.4 ± 0.3 | 45.1 ± 0.1 | 7.87 ± 0.1 | 5.73 ± 0.4 | 1.16 ± 0.01 | 9.15 ± 0.08 | 1.32 ± 0.06 | 0.18 ± 0.01 | 0.16 ± 0.02 | 0.39 ± 0.02 | 100 ± 1.3 | 185 ± 2.6 |
| Comp HLS | 8.2 ± 0.5 | 35.1 ± 0.1 | 4.34 ± 0.2 | 8.10 ± 0.3 | 1.45 ± 0.02 | 5.49 ± 0.05 | 2.59 ± 0.05 | 0.49 ± 0.02 | 0.58 ± 0.02 | 0.15 ± 0.01 | 216 ± 2.3 | 353 ± 4.7 |
| Plant Height (cm) | Stems (no. Plant−1) | Flowers (no. Plant−1) | Fruits (no. Plant−1) | Leaves (no. Plant−1) | Leaf Area (cm2 Plant−1) | |
|---|---|---|---|---|---|---|
| Control | 31.3 c | 15.8 c | 34.5 b | 30.0 c | 91.1 c | 889.1 c |
| Leonar HLS | 38.4 b | 20.2 bc | 37.2 b | 53.4 bc | 116.6 bc | 1243.0 b |
| Digest HLS | 47.2 a | 20.8 b | 59.8 a | 74.0 ab | 132.6 ab | 1387.6 b |
| Comp HLS | 50.5 a | 26.8 a | 74.4 a | 102.5 a | 148.8 a | 1730.3 a |
| Significance | *** | *** | *** | ** | *** | ** |
| Fresh Weight (g) | Dry Weight (g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Stem | Leaf | Root | Total | Stem | Leaf | Root | Total | |
| Control | 10.2 b | 37.3 b | 34.7 c | 82.2 c | 3.6 b | 14.9 b | 13.6 | 32.0 c |
| Leonar HLS | 10.8 b | 45.1 ab | 38.2 bc | 94.1 bc | 4.3 b | 18.6 ab | 15.2 | 38.1 bc |
| Digest HLS | 13.9 ab | 49.7 ab | 42.3 b | 105.9 b | 5.4 ab | 20.4 ab | 17.2 | 43.1 b |
| Comp HLS | 17.9 a | 57.4 a | 54.6 a | 129.9 a | 7.4 a | 23.7 a | 22.4 | 53.6 a |
| Significance | *** | * | * | *** | ** | * | ns | * |
| Root Length (cm) | Shoot Dry Matter (%) | R/S | WUE (g L−1) | |
|---|---|---|---|---|
| Control | 20.5 d | 57.6 a | 0.77 | 3.6 c |
| Leonar HLS | 23.3 c | 60.0 a | 0.75 | 4.8 bc |
| Digest HLS | 27.7 b | 60.1 a | 0.88 | 6.2 b |
| Comp HLS | 31.5 a | 58.1 a | 0.73 | 8.9 a |
| Significance | *** | ns | ns | *** |
| SPAD | Pn (μmol CO2 m−2 s−1) | Gs (mmol m−2 s−1) | Total Chlorophylls (µg cm−2) | |
|---|---|---|---|---|
| Control | 63.2 c | 2.38 b | 0.006 c | 85.1 c |
| Leonar HLS | 75.2 b | 3.51 b | 0.015 bc | 116.3 b |
| Digest HLS | 81.5 a | 5.60 a | 0.035 b | 134.7 ab |
| Comp HLS | 90.4 a | 7.05 a | 0.062 a | 159.9 a |
| Significance | ** | * | * | ** |
| L* (Lightness) | a* (Red/Green) | b* (Yellow/Blue) | Chroma (Saturation) | Hue Angle (Degree°) | |
|---|---|---|---|---|---|
| Control | 58.0 a | −3.0 ab | 15.0 b | 16.8 c | 161.6 |
| Leonar HLS | 43.9 ab | −6.4 b | 27.2 a | 37.9 a | 243.2 |
| Digest HLS | 37.7 b | −0.2 a | 26.1 a | 33.6 a | 295.6 |
| Comp HLS | 37.6 b | −11.9 c | 21.3 ab | 24.5 b | 331.4 |
| Significance | ** | * | * | * | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fascella, G.; Montoneri, E.; Rouphael, Y. Biowaste-Derived Humic-like Substances Improve Growth and Quality of Orange Jasmine (Murraya paniculata L. Jacq.) Plants in Soilless Potted Culture. Resources 2021, 10, 80. https://0-doi-org.brum.beds.ac.uk/10.3390/resources10080080
Fascella G, Montoneri E, Rouphael Y. Biowaste-Derived Humic-like Substances Improve Growth and Quality of Orange Jasmine (Murraya paniculata L. Jacq.) Plants in Soilless Potted Culture. Resources. 2021; 10(8):80. https://0-doi-org.brum.beds.ac.uk/10.3390/resources10080080
Chicago/Turabian StyleFascella, Giancarlo, Enzo Montoneri, and Youssef Rouphael. 2021. "Biowaste-Derived Humic-like Substances Improve Growth and Quality of Orange Jasmine (Murraya paniculata L. Jacq.) Plants in Soilless Potted Culture" Resources 10, no. 8: 80. https://0-doi-org.brum.beds.ac.uk/10.3390/resources10080080






