1. Introduction
Ultraviolet radiation, air pollutants, psychological stress, alcohol assumption, smoking, and chemical exposure are capable of inducing free radicals and reactive oxygen species on the skin. Free radicals are defined as atomic, molecular, or ionic species containing an unpaired valence electron in an atomic orbital, resulting in the highly chemical reactive properties of free radicals. Free radicals are highly unstable and have electrons available to react with various biological substances, including lipid molecules, proteins, and DNA, causing cell damage and homeostatic disruption [
1]. An excess of free radicals generates oxidative stress and damages cell membrane and lipoproteins through lipid oxidation processes.
Skin has endogenous antioxidants, such as glutathione, melanin, and enzymatic antioxidants [
2]. However, the excess formation of free radicals requires exogenous antioxidant topical application in preventing oxidative stress and enhancing DNA repair. Several studies have shown that the oxidation could be prevented by prior antioxidant treatment. Antioxidants protect the skin by reducing free-radical production. Scavenging free radicals by antioxidants can prevent skin aging. Antioxidants also have anti-inflammatory properties in preventing sunburn and protecting the skin from sun damage and photoaging. By reducing inflammation, antioxidants stimulate skin repair and correct skin damage. Free radicals can trigger the skin’s melanin production, causing skin color changes. Antioxidants prevent skin pigment generation by reducing photodamage. In addition, some antioxidants were shown to increase skin hydration to revitalize the skin [
3].
Natural oils containing unsaturated fatty acids are widely used as natural antioxidants and moisturizers to prevent skin dryness and aging. However, the topical delivery of natural oil requires a formulation acting as an oil carrier and diluent to minimize the skin irritation caused by direct contact with the oils. Therefore, further research regarding natural oil formulation and its safety and efficacy for prevention and treatment of skin diseases is required.
Moringa oleifera seed oil has a light yellow color with a mild nutty odor. Research suggested that
M. oleifera seed oil possesses a skin protecting effect.
M. oleifera seed oil was suggested to maintain the natural skin pigmentation as it possesses a mild sun protective activity [
4]. The anti-fungal activity of
M. oleifera seed oil has been reported [
5].
M. oleifera see oil has been used to alleviate earache and prevent mosquito bites. In some African countries, moringa seed oil is used in soap formulation to improve the stability of the lather and the cleaning efficiency (4).
The benefits of M. oleifera seed oil for the skin have been widely recognized. However, the research supporting its use is insufficient. The antioxidant activities and effects of skin hydration, skin color, and skin visco-elasticity of M. oleifera seed oil formulations have not been investigated. In addition, there are very limited data regarding the safe and effective dose of M. oleifera seed oil incorporated in formulations. In this study, we characterized the antioxidant activity of moringa seed oil. The chemical compositions of moringa seed oil were analyzed to validate its biological activities. A cream containing M. oleifera seed oil was formulated. The physical stability and antioxidant activity of the cream were tested. The safety and efficacy of the formulation were also reported.
3. Methods
3.1. Characterization of M. oleifera Seed Oil
3.1.1. Determination of α-Tocopherol in M. oleifera Seed Oil
The amount of α-tocopherol in
M. oleifera seed oil was determined using high-performance liquid chromatography (HPLC) [
6].
M. oleifera seed oil (1 g) was mixed with deionized water and ascorbic acid (1 g) was added as an antioxidant. Potassium hydroxide (3 mL) and ethanol (3 mL) were added, and the saponification was performed under reflux at 85 °C for 30 min. A hexane and ethyl acetate (8:2) mixture was added and vortexed. The upper hexane layer was collected, washed with deionized water to remove excess potassium hydroxide and evaporated to dryness.
The sample was then dissolved in 1 mL ethanol. The amount of α-tocopherol was determined using HPLC (Agilent 1200 Series, Santa Clara, CA, USA). The separation was achieved on Platinum (C18), 5 µm, 250 × 4.6 mm. The injection volume was 20 µL. The column temperature was 40 °C. The chromatography was performed using an isocratic elution mode in which 100% methanol was used as a mobile phase. The flow rate and detection wavelength was kept constant at 1 mL/min and 290 nm, respectively.
3.1.2. Quantification of Plant Sterols in M. oleifera Seed Oil by Gas Chromatography
Quantification of the plant sterols in
M. oleifera seed oil was performed using gas chromatography [
7]. Stock solutions of brassicasterol, campesterol, and stigmasterol at 500 ppm were prepared by dissolving each compound at 0.5 mg/mL in n-hexane. Working solutions with concentrations of 10–200 ppm were prepared by diluting the stock solutions of brassicasterol, campesterol, and stigmasterol with n-hexane and were kept at −20 °C. The plant sterol standards were derivatized by mixing 9 mL trimethylsilyl (TMS) pyridine, 3 mL hexane, and 1 mL chloroform with 100 µL of the sample. The derivatives were dried by dry nitrogen and filtered before injection.
The samples were prepared by liquid extraction and saponification. Moringa seed oil was weighed at 2.5 g. The 5-α-cholestan 50 ppm internal standard (1 mL) and ethanolic potassium hydroxide (1 mL) were added and mixed for 1 min and heated at 60 °C for 30 min. Deionized water (5 mL) and chloroform (10 mL) were added to the sample and mixed for 2 min. The upper layer was collected. The extraction was repeated by adding hexane into the lower part and mixing for 2 min. The upper layer was collected and evaporated at 50 °C to dryness.
Derivatization of the plant sterols in the moringa seed oil sample was performed by the above-mentioned method. Gas chromatography (GC) analysis was carried out by using a 6850 Series GC System (Agilent Technologies, Santa Clara, CA, USA) and a chemically bonded fused silica capillary column of methylsiloxane (HP1, 30 m × 0.32 mm i.d., 0.25 µm film thickness). The inlet temperature was 270 °C, and the detector temperature was 200 °C (1 min) with an increment of 5 °C/min to the final temperature 290 °C (14 min), at a flow rate of 0.5 mL/min, and the total run time was 33 min.
3.1.3. Determination of the Fatty Acid Composition in Moringa Seed Oil
The analysis of the fatty acid composition in
M. oleifera seed oil followed the Compendium of methods for food analysis [
8]. Moringa seed oil (1.0 g) was weighed in an Erlenmeyer flask and mixed with 30 mL of hexane:acetone (4:1
v/
v) vigorously for 1 min. The upper layer was then filtered through Whatman No. 1 filter paper containing sodium sulfate anhydrous. The extraction of fatty acid in moringa seed oil was repeated two more times. The solvent was rotary evaporated to dryness. The residue was weighed and dissolved in 5 mL of 0.5 N potassium hydroxide in methanol. Tricosanoic acid methyl ester (800 µg/mL, 1 mL), as an internal standard, was added.
The mixture was placed in a water bath shaker at 100 °C for 5 min. After the mixture was cooled, 14% borontrifluoride (BF3) in methanol (2 mL) was added. The mixture was then placed in a water bath shaker at 100 °C for 15 min. After adding saturated sodium chloride solution (10 mL) to the mixture, the supernatant was further extracted with petroleum ether (4 mL/time) until a clear solution was obtained. All extracts were collected and rotary evaporated under controlled temperature and pressure until dry. The mixture was mixed with n-heptane (3 mL) and filtered through a nylon syringe filter 13 mm diameter, 0.45 µm before analysis using Varian CP-3800 GC. Each fatty acid content was calculated by using a standard fatty acid methyl ester mixture.
3.1.4. Peroxide Value
The peroxide value of moringa seed oil was determined by the iodometric titration method 965.33 [
9]. We weighed 5 g of moringa seed oil and added 30 mL of acetic acid and chloroform (3:2). Potassium iodide (0.5 mL) was added and left for 1 min before adding 30 mL of water. A starch solution (0.5 mL) was added, and the sample was then titrated with 0.01 N sodium thiosulfate.
3.1.5. Thiobarbituric Acid Reactive Substances (TBARS) Assay
The lipid peroxidation of
M. oleifera seed oil was analyzed by the reaction with thiobarbituric acid (TBA) [
10]. Ten grams of moringa seed oil were weighed and mixed with 50 mL of deionized water. Hydrochloric acid (4 M, 2.5 mL) was added into the mixture. The sample was boiled until 50 mL of total volume was reached. The boiled sample was pipetted (5 mL), mixed with 5 mL of TBA reagent, and immersed in a water bath at 100 °C for 35 min. A blank sample was prepared by replacing the sample with deionized water. The absorbance was measured at 538 nm using a UV-visible spectrophotometer.
3.1.6. Color Measurement
Moringa seed oil color assessment was carried out by a colorimetric measurement using a spectrophotometer (Spectraflash SF600 Plus by Data Color International, Lawrenceville, NJ, USA), and the L*, a*, b*, C, h*, and ΔE coordinates, indicating the lightness, red/green coordinate, yellow/blue coordinate, chroma, hue angle, and the change in visual perception of two given colors, respectively, were analyzed.
3.2. DPPH (2,2-diphenyl-1-picrylhydrazyl) Free-Radical Scavenging Assay
The antioxidant activity of
M. oleifera seed oil was determined in terms of hydrogen donating or radical scavenging ability using the DPPH method. Moringa seed oil was diluted into 86–860 mg/mL. Moringa seed oil (500 μL) was mixed with 500 μL of 500 μM DPPH in absolute ethanol and incubated in the dark at room temperature for 30 min. After incubation, the mixture was pipetted into a 96-well plate (200 µL/well). The absorbance was measured at 517 nm using a UV-visible microplate reader (Anthos Zenyth 340, Cambridge, UK), and the mean values were obtained from triplicate experiments. Gallic acid in absolute ethanol and vitamin E were used as positive controls, and absolute ethanol was used as a negative control. In addition, vitamin E at the same concentration range was prepared and tested with a DPPH assay using the same protocol. The percentage of radical scavenging activity was calculated by the following equation.
The percentage of radical scavenging activity was plotted against the sample concentrations. The concentration of M. oleifera seed oil that decreased the DPPH to 50% of initial concentration (IC50) was calculated using GraphPad Prism 7.
3.3. Formulation of Cream Containing M. oleifera Oil
An oil-in-water cream base containing
M. oleifera seed oil was prepared using the formulation shown in
Table 1. To prepare the cream, stearic acid, stearyl alcohol, cetyl alcohol, and sorbitan ester 80 were heated to 70 °C and were then incorporated into the heated water phase (75 °C) containing propylene glycol, polysorbate 80, and deionized water. The cream base was homogenized for 3 min, and concentrated paraben was added. The pH of the cream base was adjusted by using triethanolamine. For cream containing
M. oleifera seed oil, the cream base was cooled to 50 °C and 75 g of
M. oleifera seed oil (25 g) was gradually added and mixed with the cream base under homogenization for 3 min. For the cream base, mineral oil (25 g) was mixed with the other ingredients as a control. Concentrated paraben and triethanolamine was added. The pH of the cream base was adjusted using triethanolamine.
3.4. Evaluation of the Physical Characteristics and Stability Testing of Cream Containing M. oleifera Seed Oil
The color, odor, and homogeneity of cream containing
M. oleifera seed oil were observed visually. The pH of the cream was measured using a Mettler Toledo Electrode. The viscosity of the cream was measured using a Brookfield viscometer (Model DV-III, Brokkfield, CA, USA). The physical stability of the
M. oleifera oil cream was studied with the heating–cooling cycle method for six cycles. Samples of the cream containing
M. oleifera oil were stored in glass containers for 24 h in a refrigerator and were placed in an incubator at 45 °C for another 24 h, accounting for 1 cycle [
11].
The long-term stability of
M. oleifera seed oil cream was investigated by storage of the cream at 4, 30, and 45 °C for 28 days [
12]. The appearance, color, odor, and homogeneity of
M. oleifera oil cream after the stability studies were observed by visualization. The pH and viscosity were measured at the end of each cycle and long-term storage. The rheological characteristics of the moringa seed oil cream were characterized by a rheometer (HAAKE™, Thermo Scientific, Dreieich, Germany).
3.5. Antioxidant Activity of Cream Containing M. oleifera Seed Oil
Cream containing
M. oleifera seed oil (1 g) or cream base (1 g) were extracted with 1.5 mL of absolute ethanol in a centrifuge tube by mixing vigorously for 5 min using a vortex mixer followed by 10 min-sonication. The extract was centrifuged at 7000 rpm at 25 °C for 10 min. The supernatant was collected and analyzed for antioxidant activity using a DPPH assay. The DPPH assay was carried out by mixing 500 µL of the cream extract with 500 µL of the DPPH solution. The mixture was kept in the dark at room temperature for 30 min. The absorbance was measured at 517 and 600 nm (as a reference wavelength) using a UV-visible microplate reader (Anthos Zenyth 340, Cambridge, UK), and the mean values were obtained from triplicate experiments. The percentage of radical scavenging activity of the cream was calculated by the following equation.
3.6. Skin Irritation Test
All human research studies were approved by the Human Research Ethics Committee of Srinakharinwirot University (013/2557). Thirty-two volunteers aged 18 to 65 years old (averaged 31.9 ± 12.3 years old) were recruited into the skin irritation study. Subjects were excluded if they had contact dermatitis, or any allergic reactions on the tested region that may interfere with the test. Subjects who had received any anti-allergic medications for treating allergic reactions caused by sunscreen cream, moisturizing cream, and antiaging cream were also excluded. All participants were required to sign an informed consent agreement.
The skin irritation test used in this study was modified from a patch test [
13]. After recruitment, the subjects were informed that they were allowed to shower as normal, but they were not allowed to apply any other cosmetics or drugs at the site of the sample applications prior to the test for 24 h. The test products including cream containing moringa seed oil and cream base were applied at different areas at the outer side of left arms under an occlusive patch for 48 h. The patch sites were graded using a skin irritation grading scale according to the International Contact Dermatitis Research Group (ICDRG) [
13].
The grading scale included doubtful (?+); a mild reaction, possible erythema, infiltration, and papules (+); a strong reaction, erythema, infiltration, papules, and vesicles (++); and a very strong reaction, intense erythema, infiltration, and coalescing vesicles (+++). The occlusion was removed after 48 h of application and the affected areas were observed and evaluated immediately according to the criteria. The next readings were done again 72, 96, 120, 144, 168, 192, 216, and 240 h after application. The further investigation of the moringa seed oil cream efficacy was continued only after less than 10% of all subjects had positive readings.
3.7. Skin Hydration, Erythema, Melanin Value, and Elasticity
The effects of moringa seed oil cream on the skin hydration, erythema, melanin content, and elasticity were investigated and compared to those of the cream base on its own. Prior to the study, the subjects were asked to wash their arms and wait for 15 min under controlled temperature (25 °C) and humidity (40–60%RH). The skin hydration, erythema, melanin contents, and elasticity of all 32 subjects were determined using a Corneometer®, Mexameter® MX 18, and Cutometer®. The measurements were performed at the marked area, 8 cm away from the front of the elbow and had an area of 3 × 3 cm. The subjects were informed to apply 0.2 g of Moringa seed oil cream and cream base twice a day, at 7–9 a.m. and 7–9 p.m. routinely for 4 weeks. During the experimental period, the subjects were asked to avoid sunlight and the application of any other moisturizers. The skin analysis was conducted after sample application to skin for 1, 2, 3, and 4 weeks.
After washing the arm at the sample application sites, the subjects were kept in a room with controlled temperature (25 °C) and relative humidity of 40–60%. The skin hydration test was performed using a Corneometer®, which indicated the hydration level of the superficial layers of the skin (stratum corneum) via measurement of the skin’s dielectric properties. The skin hydration effects of moringa seed oil cream and cream base were evaluated in terms of the difference in skin hydration (%) compared with the baseline and the number of participants with sufficiently hydrated skin. Mexameter® MX 18 was used to measure the hemoglobin and melanin, which are responsible for the redness and color of the skin, respectively.
The absorption and reflection intensity of light at wavelengths of 568 and 660 nm, respectively, were determined and computed as erythema values. A melanin value was computed from the intensities of the absorbed and reflected light at 660 and 880 nm, respectively. The skin elasticity was measured using a Cutometer
®. The skin elasticity was reported as the final distension (U
f) and immediate retraction (U
r). The Ur/Uf parameter was used to characterize the elastic property of the skin. The curves from the skin visco-elasticity measurement were analyzed using the Software Cutometer
®MPA 580 to obtain the final distension of the first curve (U
f) and the ratio of elastic recovery to the total deformation (U
r/U
f) parameters [
14]. All measurements were performed in triplicate.
3.8. Satisfaction Regarding Cream Containing M. oleifera Seed Oil
The satisfaction regarding the cream containing M. oleifera seed oil was measured by means of a questionnaire. Healthy volunteers reported the consumer acceptability scores on a five-point hedonic scale (scale 1: extremely dislike, scale 2: slightly dislike, scale 3: neither like nor dislike, scale 4: slightly like, and scale 5: extremely like) after using blinded cream base or M. oleifera seed oil cream. Participants evaluated their satisfaction regarding the color, smoothness, skin hydration feeling, skin absorption, spreadability, and odor.
3.9. Statistical Analysis
Statistical analysis of the data was performed using a one-way analysis of variance (ANOVA), followed by Newman–Keuls as a post-hoc test to assess the significance of differences (GraphPad Prism, La Jolla, CA, USA). To determine the significance of the difference between the means of two groups, a t-test was performed. In all cases, a value of p < 0.05 was considered statistically significant.
5. Discussion
M. oleifera seed oil obtained from cold-pressed extraction contains several bioactive compounds that have antioxidant and moisturizing properties in topical use. The amount of α-tocopherol in cold-pressed
M. oleifera seed oil was found to be slightly higher than the average amount of α-tocopherol previously reported. Anwar et al. reported that Soxhlet extraction of
M. oleifera seed oil from Pakistan using hexane as a solvent yielded 134.42 mg/kg of α-tocopherol [
17]. Ogunsina et al. showed that cold-pressed moringa seed oil exhibited higher total tocopherol contents compared with hexane-extracted moringa seed oil, suggesting that the extraction method of moringa seed oil influenced the α-tocopherol yield [
18]. α-Tocopherol is a form of vitamin E, which is a major naturally antioxidant used for protecting skin from the effects of oxidative stress, including photoaging [
19].
The plant sterol components contained in cold-pressed moringa seed oil were found to be brassicasterol, campesterol, and stigmasterol. Campesterol and stigmasterol were also major sterol components reported by many researchers [
20,
21,
22,
23]. Bezerra and Filho revealed that the major plant sterols characterized by GC were β-sitosterol, campesterol, stigmasterol, and brassicasterol. The concentrations of plant sterols, except for brassicasterol, found in this study were lower compared with in the moringa seed oil reported in other studies [
24]. The reported content of each plant sterol were different depending on the temperature and plant location, harvest time, fertilization, genetic characters, and growing conditions [
25].
The fatty acid profiles of
M. oleifera seed oil consisted of a high level of oleic acid followed by palmitic acid, and stearic acid. These results were consistent with the fatty acid profiles of moringa seed oil in other regions showing that oleic acid was the most abundant unsaturated fatty acid found in moringa seed oil [
26]. Oleic acid is monounsaturated fatty acid shown to increase the skin permeability. Correa et al. reported that trans-epidermal water loss and fluorescein penetration increased with increased doses of oleic acid.
Oleic acid penetrated into the skin and disordered stratum corneum lipids and disrupted the skin barrier function by having an affinity between oleic acid and stratum corneum lipids [
27]. In addition to α-tocopherol, several studies have reported that unsaturated fatty acids, including oleic acid and α-linoleic acid, were shown to have antioxidant activity [
28,
29]. According to the chemical compositions of
M. oleifera seed oil, the oil is recommended for use in pharmaceutical preparations, particularly for skin hydration.
M. oleifera seed oil contains fatty acids and plant sterols, which are prone to lipid peroxidation. Therefore, it was necessary to determine the peroxide value to indicate the rancidity of the oil. The TBARS value, defined as the amount of malondialdehyde (MDA) in 1 kg of
M. oleifera seed oil, was used to analyze the lipid peroxidation of the oil. The TBARS value of
M. oleifera seed oil reported in this study was 0.17 mg MDA/kg oil, which was lower than 1 mg MDA/kg oil, indicating a low extent of lipid peroxidation according to the Association of Official Agricultural Chemists (AOAC) standard criteria [
30]. The results suggest that
M. oleifera seed oil is qualified for use as a pharmaceutical or cosmetic active ingredient.
A DPPH assay was used to test the free-radical scavenging activity of M. oleifera seed oil, which donated electrons or hydrogens to DPPH to neutralize the free radicals. In the present study, the IC50 value of moringa seed oil was 121.9 mg/mL, whereas vitamin E had an IC50 value of 110.4 mg/mL. The antioxidant activity of moringa seed oil likely resulted from the presence of α-tocopherol and different types of unsaturated fatty acids in the oil. The antioxidant activity may prevent lipid peroxidation and protect moringa seed oil from rancidity.
The application of pure moringa seed oil directly to the skin may cause skin irritation and/or sensitization in normal individuals especially for those with sensitive skin. Therefore, the inclusion of moringa seed oil in the cream was an alternative method of using an essential oil for therapeutic or cosmetic uses. In this study, cream containing an effective concentration of M. olerifera seed oil was formulated. The moringa seed oil cream had a light yellow color, nutty odor, and homogeneous texture. The pH of the moringa seed oil cream was close to that of human skin, and the viscosity yielded an easily spreadable cream formulation.
The rheology of the cream after the heating–cooling cycle stability study and long-term stability study (4 weeks) showed pseudoplastic flow. The cream flew instantaneously upon application of stress and displayed shear thinning behavior without a yield stress. Therefore, when the cream was applied to the skin, the increase in the shear rate may result in less resistance to flow and the release of moringa seed oil to the skin [
31]. Normally, emulsion is a thermodynamically unstable system consisting of immiscible oil and water mixed together. The oil-in-water cream contains oil droplets dispersed in an aqueous phase.
Coalescence, flocculation, creaming, and breaking are unstable states of the common cream/emulsion. The stability of moringa seed oil cream was previously studied. The results showed that excipients, such as polysaccharides and emulsifiers influenced the physicochemical properties and stability of the emulsion. In addition, the viscosity of the emulsion also affected its stability. This study demonstrated a new cream/emulsion formulation containing M. oleifera seed oil as the active ingredient. The 25% of moringa seed oil was found to be the high concentration of moringa seed oil incorporated into the cream/emulsion formulation [
32,
33]. The high concentration of moringa seed oil could result in an unstable emulsion. However, the cream containing moringa seed oil developed in this study had an unchanged appearance, pH, viscosity, and rheology throughout the stability studies.
According to the antioxidant activity of moringa seed oil, moringa seed oil cream may protect the skin by limiting the free-radical production, preventing oxidative stress, and enhancing DNA repair upon exposure to the air, UV radiation, pollutants, and chemicals that cause skin damage [
34,
35]. In addition, moringa seed oil cream may provide skin with hydration and increase the moisture retention to aid in revitalizing the skin.
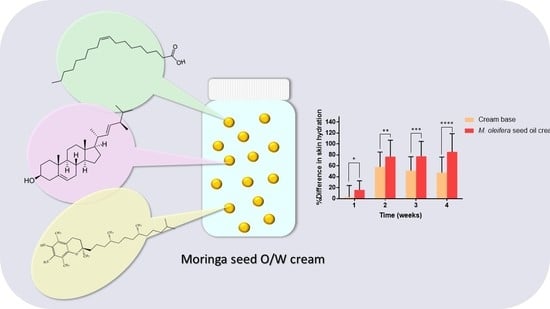
The moringa seed oil cream developed in this study passed the standard of a patch test suggesting that moringa seed oil cream is safe for use on human skin. A cream formulation was selected as the drug delivery system for the moringa seed oil. The cream base itself also had a moisturizing effect for the skin as shown in
Figure 6. Therefore, cream is a preferred vehicle because it provides a good delivery of the active ingredient, and, at the same time, the vehicle itself also promotes better skin hydration [
36]. In this study, an oil-in-water cream was developed because it provides a more pleasant application compared to a water in oil cream [
37]. The oil-in-water cream was therefore more acceptable to the consumers to apply the product more frequently. In addition, an oil-in-water skin cream spreads over the skin more easily, which could result in enhanced absorption of the active moringa seed oil.
The skin hydration level of the subjects applying moringa seed oil cream was significantly increased compared with those using the cream base. Moringa seed oil increased the skin hydration by creating a hydrophobic barrier over the skin and blocked dehydration. Oleic acid in the moringa seed oil disrupted the skin barrier, increased the molecular interactions of the moringa seed oil with stratum corneum lipids and enhanced the oil penetration efficiency into the epidermis [
38]. The skin erythema was significantly reduced after the application of moringa seed oil. Erythema was a result of skin inflammation and irritation. The level of erythema values was directly related to the hemoglobin content in the skin.
The reduction in erythema values after the application of moringa seed oil cream suggests that the cream is safe for use in cosmetic and pharmaceutical applications without causing any significant skin irritation [
39]. These results were confirmed by the results of the patch test showing no irritation to the skin and that the formulation was appropriate for human use [
40]. The melanin value and visco-elasticity were not increased with the use of moringa seed oil cream, indicating that moringa seed oil cream did not affect the melanin content and elasticity of the skin in a 4-week application. The satisfaction score of participants revealed that the consumers were satisfied with the color, smoothness, and skin hydration feeling of the moringa seed oil cream. Therefore, it might not be necessary to add any coloring agents to the cream. However, a flavoring agent was recommended to be included in the cream formulation.