Influence of Vitamins on Secondary Reactive Oxygen Species Production in Sera of Patients with Resectable NSCLC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Blood Sampling
2.3. Assay for SOS Production Analysis
2.4. Statistical Analysis
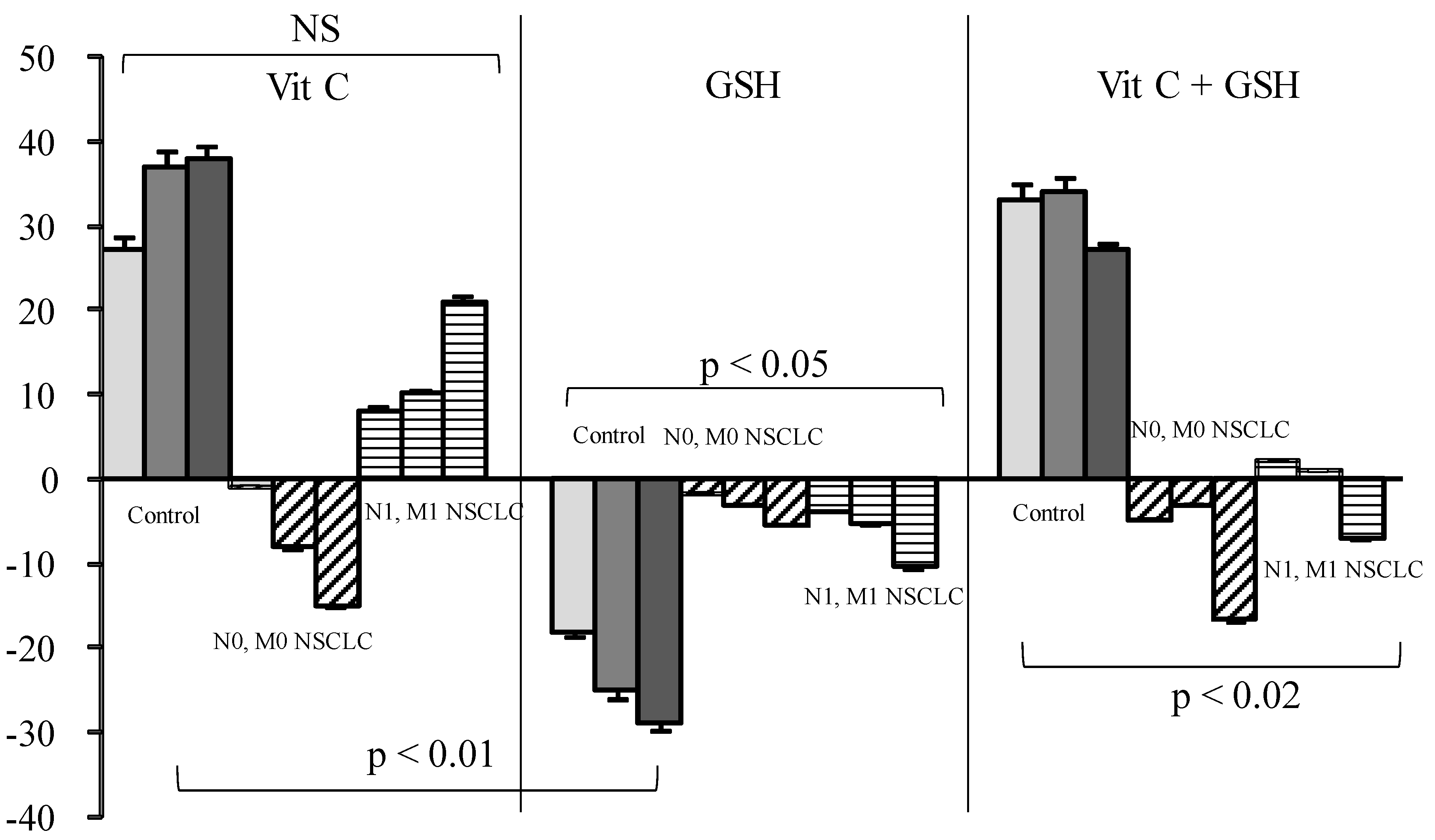
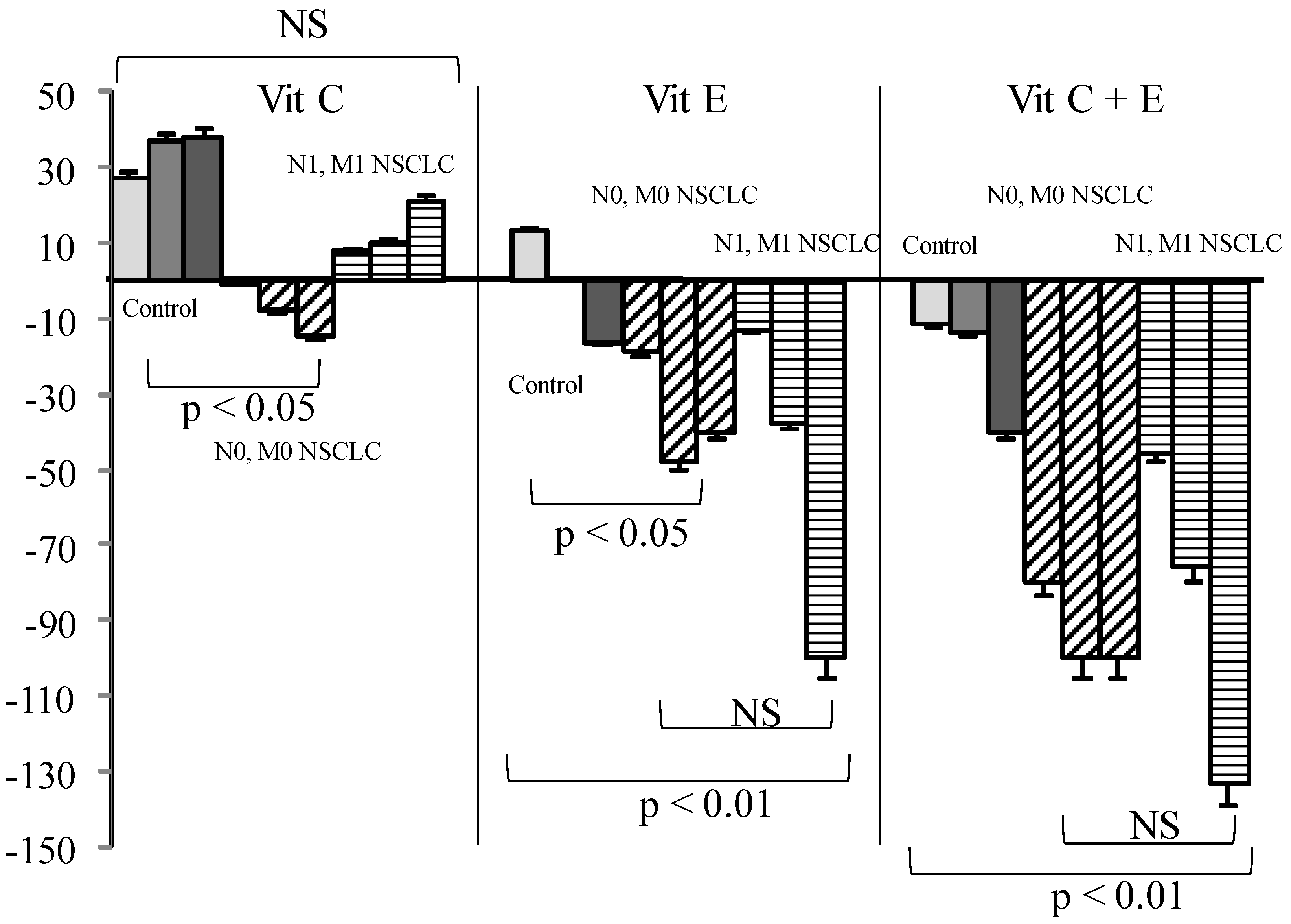
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
Abbreviations
| 1O2 | Singlet oxygen |
| ADK | Adenocarcinoma |
| AUC | Area Under the Curve |
| DCF | Dichlorofluorescein (oxidized, fluorescent) |
| DCF-DA | Dichlorofluorescein Diacetate |
| DCFH | Dichlorofluorescein (reduced, non fluorescent) |
| FEV 1 | Forced expiratory volume in one second |
| GSH | Reduced glutathione |
| Met | Metastase |
| NSCLC | Non-small cell lung cancer |
| RB | Rose Bengal |
| ROS | Reactive Oxygen Species and peroxides |
| SCC | Squamous cell carcinoma |
| SD | Standard Deviation |
| SOS | Secondary Reactive Oxygen Species and peroxides |
| Vit C | L-ascorbic acid, Vitamin C |
| Vit D | Vitamine D2 (ergocalciferol) and D3 (cholecalciferol) (25-OH) |
| Vit E | Alpha tocopherol, Vitamin E |
References
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer: A double-edged sword with therapeutic potential. Oxid. Med. Cell. Longev. 2009, 3, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Azeez, O.I.; Saba, A.B. Interactions between reactive oxygen species and cancer: The roles of natural dietary antioxidants and their molecular mechanisms of action. Asian Pac. J. Cancer Prev. 2009, 10, 535–544. [Google Scholar] [PubMed]
- Zuo, L.; Hallman, A.; Yousif, M.; Chien, M. Oxidative stress, respiratory muscle dysfunction, and potential therapeutics in chronic obstructive pulmonary disaese. Front. Biol. 2012, 7, 506–513. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species—The good, the bad and the ugly. Acta Physiol. (Oxf.) 2015, 214, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Kanofsky, J.R. Singlet oxygen production by biological systems. Chem. Biol. Interact. 1989, 70, 1–28. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Kon, T.; Tanigawa, T.; Hayamizu, K.; Shen, M.; Tsuji, T.; Naito, Y.; Yoshikawa, T. Singlet oxygen quenching activity of human serum. Redox. Rep. 2004, 9, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Tanielian, C.; Mechin, R.; Seghrouchni, R.; Schweitzer, C. Mechanistic and kinetic aspects of photosensitization in the presence of oxygen. Photochem. Photobiol. 2000, 71, 12–19. [Google Scholar] [CrossRef]
- Bigot, E.; Bataille, R.; Patrice, T. Increased singlet oxygen-induced secondary ROS production in the serum of cancer patients. J. Photochem. Photobiol. B 2012, 107, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Douillard, S.; Rozec, B.; Bigot, E.; Aillet, L.; Patrice, T. Secondary reactive oxygen species production after PDT during pulmonary tumor growth in sera of nude mice. Photodiagnosis Photodyn. Ther. 2013, 10, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Choi, K.; Kim, J.; Bae, W.; Kim, S.; Sohn, C. Relationship between inflammation biomarkers, antioxidant vitamins, and bone mineral density in patients with metabolic syndrome. Nutr. Res. Pract. 2011, 5, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Farbstein, D.; Blum, S.; Pollak, M.; Asaf, R.; Viener, H.L.; Lache, O.; Asleh, R.; Miller-Lotan, R.; Barkay, I.; Star, M.; et al. Vitamin E therapy results in a reduction in HDL function in individuals with diabetes and the haptoglobin 2-1 genotype. Atherosclerosis 2011, 219, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Suh, N.; Kong, A.N. Does vitamin E prevent or promote cancer? Cancer Prev. Res. (Phila.) 2012, 5, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Vergnaud, A.C.; Galan, P.; Arnaud, J.; Favier, A.; Faure, H.; Huxley, R.; Hercberg, S.; Ahluwalia, N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am. J. Clin. Nutr. 2009, 90, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Omenn, G.S.; Goodman, G.E.; Thornquist, M.D.; Balmes, J.; Cullen, M.R.; Glass, A.; Keogh, J.P.; Meyskens, F.L., Jr.; Valanis, B.; Williams, J.H., Jr.; et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J. Natl. Cancer Inst. 1996, 88, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Ascorbate function and metabolism in the human erythrocyte. Front. Biosci. 1998, 3, d1–d10. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione, ascorbate, and cellular protection. Cancer Res. 1994, 54, 1969s–1975s. [Google Scholar] [PubMed]
- Ciocoiu, M.; Badescu, M.; Paduraru, I. Protecting antioxidative effects of vitamins E and C in experimental physical stress. J. Physiol. Biochem. 2007, 63, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Olivier, D.; Douillard, S.; Lhommeau, I.; Bigot, E.; Patrice, T. Secondary oxidants in human serum exposed to singlet oxygen: The influence of hemolysis. Photochem. Photobiol. Sci. 2009, 8, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Diaz, P.T.; Best, T.M.; Stimpfl, J.N.; He, F.; Zuo, L. Molecular characterization of redox mechanisms in allergic asthma. Ann. Allergy Asthma Immunol. 2014, 113, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muniesa, P.; Garcia-Gerique, L.; Quintero, P.; Arriaza, S.; Lopez-Pascual, A.; Martinez, J.A. Effects of Hyperoxia on Oxygen-Related Inflammation with a Focus on Obesity. Oxid. Med. Cell. Longev. 2015, 2015, 8957827. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox. Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.C.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Introduction to the molecular basis of cancer metabolism and the Warburg effect. Mol. Biol. Rep. 2015, 42, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Esme, H.; Cemek, M.; Sezer, M.; Saglam, H.; Demir, A.; Melek, H.; Unlu, M. High levels of oxidative stress in patients with advanced lung cancer. Respirology 2008, 13, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371. [Google Scholar] [PubMed]
- Foong, R.E.; Zosky, G.R. Vitamin D deficiency and the lung: Disease initiator or disease modifier? Nutrients 2014, 5, 2880–2900. [Google Scholar] [CrossRef] [PubMed]
- Anic, G.M.; Weinstein, S.J.; Mondul, A.M.; Mannisto, S.; Albanes, D. Serum vitamin D, vitamin D binding protein, and lung cancer survival. Lung Cancer 2014, 86, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.; Diaz-Munoz, M.; Garcia-Gaytan, A.C.; Mendez, I. Mechanistic Effects of Calcitriol in Cancer Biology. Nutrients 2015, 7, 5020–5050. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Buchholz, J.; Keller, M.; Bettini, G.; Valenti, P.; Kaser-Hotz, B. Multimodal therapeutic approach and interdisciplinary challenge for the treatment of unresectable head and neck squamous cell carcinoma in six cats: A pilot study. Vet. Comp. Oncol. 2013, 11, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Redox biology of exercise. Oxid. Med. Cell. Longev. 2012, 2012, 407978. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Hutton, B.; Ng, T.; Shorr, R.; Clemons, M. Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review. Oncologist 2015, 20, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.R.; Nielsen, J.B.; Nielsen, F.; Grandjean, P. Antioxidative enzyme activities in human erythrocytes. Clin. Chem. 1997, 43, 562–568. [Google Scholar] [PubMed]
- Anderson, D.; Phillips, B.J. Comparative in vitro and in vivo effects of antioxidants. Food Chem. Toxicol. 1999, 37, 1015–1025. [Google Scholar] [CrossRef]
- Babaev, V.R.; Li, L.; Shah, S.; Fazio, S.; Linton, M.F.; May, J.M. Combined vitamin C and vitamin E deficiency worsens early atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Meister, A. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: Ascorbate spares glutathione and protects. Proc. Natl. Acad. Sci. USA 1991, 88, 4656–4660. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Yu, T.W.; Phillips, B.J.; Schmezer, P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat. Res. 1994, 307, 261–271. [Google Scholar] [CrossRef]
- Galley, H.F.; Howdle, P.D.; Walker, B.E.; Webster, N.R. The effects of intravenous antioxidants in patients with septic shock. Free Radic. Biol. Med. 1997, 23, 768–774. [Google Scholar] [CrossRef]
- Guerin, P.; Bigot, E.; Patrice, T. Evidence for antioxidants consumption in the coronary blood of patients with an acute myocardial infarction. J. Thromb. Thrombolysis 2013, 35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hafer, K.; Iwamoto, K.S.; Schiestl, R.H. Refinement of the dichlorofluorescein assay for flow cytometric measurement of reactive oxygen species in irradiated and bystander cell populations. Radiat. Res. 2008, 169, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Summa, D.; Spiga, O.; Bernini, A.; Venditti, V.; Priora, R.; Frosali, S.; Margaritis, A.; di Giuseppe, D.; Niccolai, N.; di Simplicio, P. Protein-thiol substitution or protein dethiolation by thiol/disulfide exchange reactions: The albumin model. Proteins 2007, 69, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Krishnan, S. Vitamin E Analogs as Radiation Response Modifiers. Evid. Based Complement. Alternat. Med. 2015, 2015, 741301. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Spiegelman, D.; Hunter, D.J.; Albanes, D.; Bergkvist, L.; Buring, J.E.; Freudenheim, J.L.; Giovannucci, E.; Goldbohm, R.A.; Harnack, L.; et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: A pooled analysis of prospective cohort studies. Cancer Causes Control 2010, 21, 1745–1757. [Google Scholar] [CrossRef] [PubMed]

| N | Whole | N | Women | N | Men | |
|---|---|---|---|---|---|---|
| Age | 61.3 | 60.7 | 61 | |||
| Whole cohort | 38 | 0.84 (0.45) | 10 | 0.79 (0.36) | 28 | 0.82 (0.46) |
| Whole N0Mo | 23 | 0.83 (0.2) | 4 | 0.63 (0.2) | 19 | 0.87 (0.2) |
| Whole N1 or M1 | 15 | 0.76 (0.2) | 5 | 0.9 (0.3) | 10 | 0.68 (0.4) |
| ADK | 19 | 0.9 (0.5) | 8 | 0.83 (0.4) | 11 | 0.88 (0.6) |
| ADK N0M0 | 11 | 0.87 (0.6) | 4 | 0.6 (0.47) | 7 | 1.07 (0.7) |
| ADK N1 or M1 | 8 | 0.84 (0.1) | 4 | 1.04 (0.3) | 4 | 0.65 (0.17) |
| SCC | 19 | 0.77 (0.35) | 2 | 0.63 (0.17) | 17 | 0.78 (0.4) |
| SCC N0M0 | 12 | 0.78 (0.4) | 0 | - | 12 | 0.72 (0.2) |
| SCC N1 or M1 | 7 | 0.69 (0.17) | 2 | 0.62 | 5 | |
| Controls | 50 | 0.85 (0.23) | 0.83 (0.21) | 0.77 (0.24) |
| N | n | Dead or Evolutive | CR | <80 | FEV 1 80 < M < 100 | 100 | |
|---|---|---|---|---|---|---|---|
| Whole | 38 | 10 | 0.85 (0.6) | 0.7 (0.35) | 0.87 (0.57) | 0.8 (0.34) | 0.8 (0.3) |
| ADK | 19 | 5 | 1.02 (0.9) | 0.83 (0.36) | 1.46 (0.9) | 0.82 (0.4) | 0.64 (0.14) |
| SCC | 19 | 4 | 0.65 (0.15) | 0.87 (0.4) | 0.75 (0.4) | 0.64 (0.06) | 0.90 (0.3) |
| Vitamin D | Ratio (Control) | |
|---|---|---|
| Control | 32.2 | 0.8 (1) |
| Women | 25.5 (0.05) | 0.83 |
| Men | 26.9 (0.04) | 0.77 |
| All K | 14.12 (10) | 0.84 |
| All K W | 13 (9.9) | 0.79 |
| All K M | 14.51 (11) | 0.82 |
| All meta − | 11.75 (6.5) | 0.83 |
| All meta + | 20.26 (15) | 0.76 |
| ADK | 16.24 (13) | 0.9 |
| ADK meta − | 11.42 (8.3) | 0.86 |
| ADK meta + | 22.51 (15) | 0.84 |
| SCC | 11.24 (5.3) | 0.77 |
| SCC meta − | 12.76 (5.1) | 0.78 |
| SCC meta + | 7.2 (5.3) | 0.69 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patrice, T.; Rozec, B.; Sidoroff, A.; Blanloeil, Y.; Despins, P.; Perrigaud, C. Influence of Vitamins on Secondary Reactive Oxygen Species Production in Sera of Patients with Resectable NSCLC. Diseases 2016, 4, 25. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases4030025
Patrice T, Rozec B, Sidoroff A, Blanloeil Y, Despins P, Perrigaud C. Influence of Vitamins on Secondary Reactive Oxygen Species Production in Sera of Patients with Resectable NSCLC. Diseases. 2016; 4(3):25. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases4030025
Chicago/Turabian StylePatrice, Thierry, Bertrand Rozec, Alexis Sidoroff, Yvonnick Blanloeil, Philippe Despins, and Christian Perrigaud. 2016. "Influence of Vitamins on Secondary Reactive Oxygen Species Production in Sera of Patients with Resectable NSCLC" Diseases 4, no. 3: 25. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases4030025





