Blood Pressure and Oxidative Stress among U.S. Adults Exposed to Lead in Military Environments—A Preliminary Study
Abstract
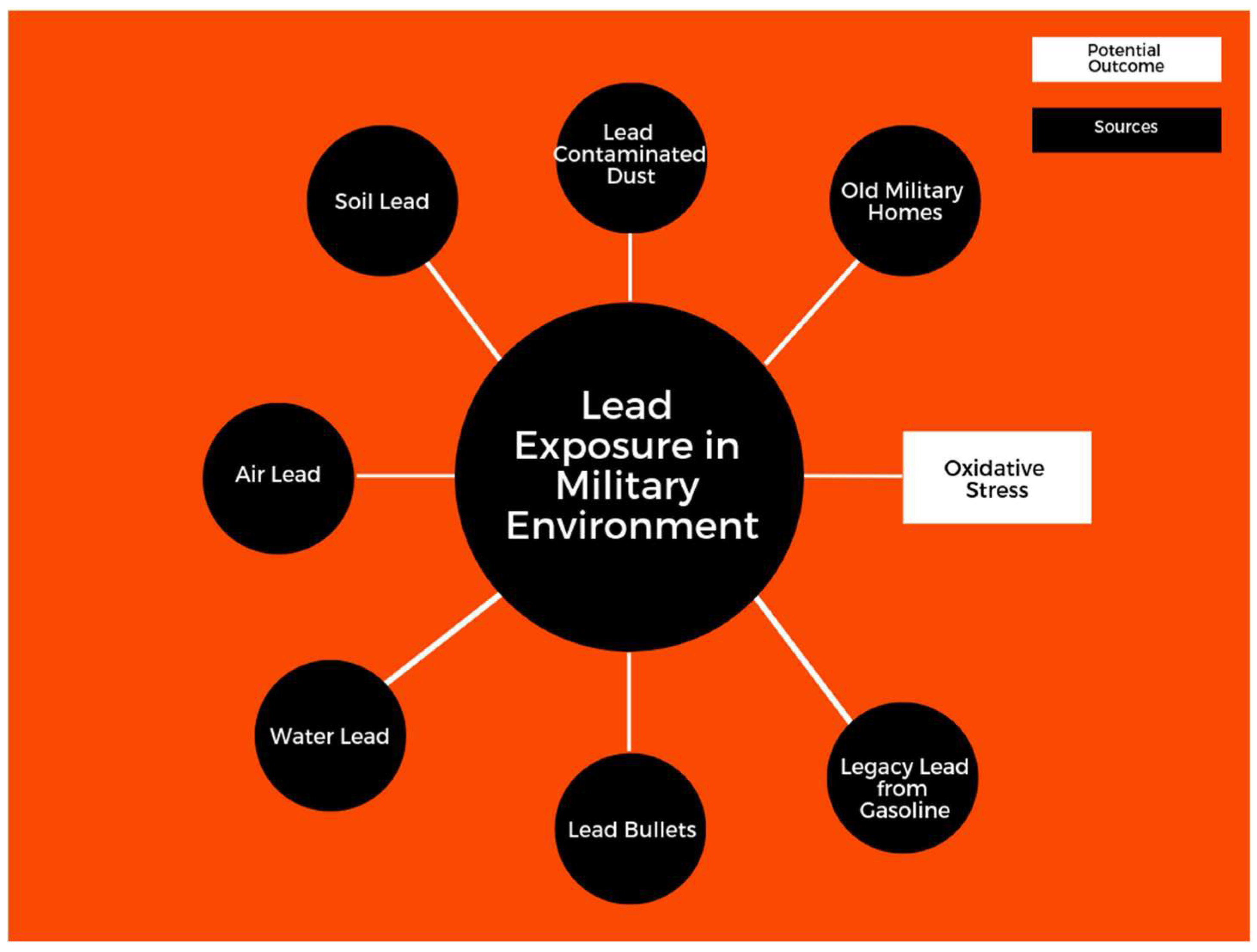
:1. Introduction
2. Materials and Methods
2.1. Study Hypothesis
2.2. Research Design
2.3. Statistical and Analytical Approaches
3. Results
3.1. Sociodemographic and Clinical Markers
3.2. Associations between BLLs and SBP, DBP, and GGT in Adults Who Have Served in Military Environments and the U.S. General Adult Population
4. Discussion
4.1. Lead and Exposure in Military Environments
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naicker, N.; de Jager, P.; Naidoo, S.; Mathee, A. Is there a relationship between lead exposure and aggressive behavior in shooters? Inter.J. Environ. Res. Pubulic Health 2018, 15, 1427. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E.; Armijos, R.X.; Weigel, M.M.; Filippelli, G.M.; Sayegh, M.A. Cardiovascular-related outcomes in US adults exposed to lead. Int.J. Environ. Res. Pubulic Health 2018, 15, 759. [Google Scholar] [CrossRef] [PubMed]
- Cory-Schlecta, D.; Schaumburg, H. Lead, inorganic. Experiment. Clin. Neurotoxicol. 2000, 2, 708–720. [Google Scholar]
- Filippelli, G.; Adamic, J.; Nichols, D.; Shukle, J.; Frix, E. Mapping the Urban Lead Exposome: A Detailed Analysis of Soil Metal Concentrations at the Household Scale Using Citizen Science. 2018. Available online: https://scholar.google.com.hk/scholar?hl=zh-CN&as_sdt=0%2C5&q=Mapping+the+Urban+Lead+Exposome%3A+A+Detailed+Analysis+of+Soil+Metal+Concentrations+at+the+Household+Scale+Using+Citizen+Science&btnG= (accessed on 21 September 2018).
- Mielke, H.W.; Laidlaw, M.A.S.; Gonzales, C. Lead (pb) legacy from vehicle traffic in eight california urbanized areas: continuing influence of lead dust on children’s health. Sci. Total. Environ. 2010, 408, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, M.A.S.; Filippelli, G.; Mielke, H.; Gulson, B.; Ball, A.S. Lead exposure at firing ranges—A review. Environ. Health 2017, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C.; Stiles, K.M.; Needleman, H.L. Low-level lead exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics 1992, 90, 855–861. [Google Scholar] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease: A systematic review. Environ. Health Perspect. 2007, 6, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E.; Armijos, R.X.; Weigel, M.M.; Filippelli, G.; Sayegh, M.A. Hepatobiliary-related outcomes in US adults exposed to lead. Environments 2018, 5, 46. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Lead exposure and oxidative stress—A life course approach in U.S. adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71. [Google Scholar] [CrossRef]
- Mishra, K. Lead exposure and its impact on immune system: A review. Toxicol. In Vitro 2009, 23, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, M.E.; Gwiazda, R.H.; Smith, D.R. Lead poisoning of seabirds: Environmental risks from leaded paint at a decommissioned military base. Environ. Sci. Technol. 2003, 37, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Frimer, R.; Meyer, R.; Derazne, E.; Chodick, G. Lead exposure in military outdoor firing ranges. Mil. Med. 2016, 181, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Rocha, E.D.; Sarkis, J.E.S.; de Fátima, H.; Carvalho, M.; dos Santos, G.V.; Canesso, C. Occupational exposure to airborne lead in Brazilian police officers. Inter. J. Hyg. Environ. Health 2014, 217, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Blomhoff, R.; Jacobs, D.R. Review is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic. Res. 2004, 38, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-S.; Yang, J.-H.; Chun, B.-Y.; Kam, S.; Jacobs, D.R., Jr.; Lee, D.-H. Is serum γ-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic. Biol. Med. 2004, 37, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Lim, J.-S.; Song, K.; Boo, Y.; Jacobs, D.R. Graded associations of blood lead and urinary cadmium concentrations with oxidative-stress–related markers in the US population: Results from the Third National Health and Nutrition Examination Survey. Environ. Health Perspect. 2006, 114, 350. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, A.; Ruggiero, C.; Polidori, M.C.; Mecocci, P. Potential markers of oxidative stress in stroke. Free Radic. Biol. Med. 2005, 39, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D.; Hodgson, M.J.; Bromet, E.J.; Dew, M.A.; Connell, M.M. Occupational lead exposure and blood pressure. Occup. Environ. Med. 1987, 44, 744–748. [Google Scholar] [CrossRef]
- Smoley, B.A.; Smith, N.L.; Runkle, G.P. Hypertension in a population of active duty service members. J. Am. Board Fam. Med. 2008, 21, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Harlan, W.R.; Landis, J.R.; Schmouder, R.L.; Goldstein, N.G.; Harlan, L.C. Blood lead and blood pressure: Relationship in the adolescent and adult US population. JAMA 1985, 253, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.; Thijs, L.; Den Hond, E.M.; Roels, H.A.; Staessen, J.A. An epidemiological re-appraisal of the association between blood pressure and blood lead: A meta-analysis. J. Hum. Hypertens. 2002, 16, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, R.M.; Pemberton, M.R.; Lane, M.E.; Hourani, L.L.; Mattiko, M.J.; Babeu, L.A. Substance use and mental health trends among US military active duty personnel: Key findings from the 2008 DoD Health Behavior Survey. Mil. Med. 2010, 175, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.; Ryan, M.A.K.; Wingard, D.L.; Patterson, T.L.; Slymen, D.J.; Macera, C.A. Millennium Cohort Study Team Cigarette smoking and military deployment: A prospective evaluation. Am. J. Prev. Med. 2008, 35, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Todd, A.C. Direct measurement of lead in bone a promising biomarker. JAMA 1994, 271, 239–240. [Google Scholar] [CrossRef] [PubMed]
| Served in Armed Forces N = 2391 | Did Not Serve in Armed Forces N = 22,747 | |
|---|---|---|
| Mean blood lead level (BLL) (95% CI) | 1.83 (1.68–1.98) | 1.33 (1.27–1.38) |
| Mean age (95% CI) | 59.47 (58.52–60.42) | 44.64 (44.08–45.21) |
| Gender percent | Men 91.9% Women 8.10% | Men 43.68% Women 56.32% |
| Education percent 1 = Less than ninth grade 2 = 9–12th grade—no diploma 3 = High school graduate/GED or equivalent 4 = Some college or associate degree 5 = College graduate or above | 1 = 1.89% 2 = 7.57% 3 = 22.69% 4 = 39.40% 5 = 28.43% | 1 = 6.08% 2= 11.01% 3 = 21.25% 4 = 31.17% 5 = 30.49% |
| Ethnicity percent 1 = Mexican-American 2 = Other Hispanic 3 = Non-Hispanic White 4 =Non-Hispanic Black 5 = Other race, including multiracial | 1 = 2.88% 2 = 2.71% 3 = 78.09% 4 = 11.34% 5 = 4.98% | 1 = 9.50% 2 = 6.29% 3 = 64.08% 4 = 11.64% 5 = 8.48% |
| Smoking percent (95% CI) | 62.91% (59.81–65.90) | 41.41% (40.10–42.72) |
| Alcohol percent (95% CI) | 87.44% (85.35–89.27) | 75.77% (74.07–77.38) |
| Systolic blood pressure (SBP) | 126.71 (125.67–127.74) | 120.69 (120.19–121.18) |
| Diastolic blood pressure (DBP) | 69.44 (68.64–70.23) | 69.59 (69.05–70.13) |
| Gamma-glutamyl transferase (GGT) | 31.02 (28.79–33.25) | 26.05 (25.42–26.69) |
| Variables | ln BPb Adjusted (95% CI) + | p-Value |
|---|---|---|
| SBP | 0.299 (−0.016, 0.615) | 0.063 |
| DBP | 0.100 (−0.108, 0.308) | 0.339 |
| GGT | 0.052 (−0.30, 0.133) | 0.208 |
| Variables | ln BPb Adjusted (95% CI) + | p-Value |
|---|---|---|
| SBP | 0.238 (0.122, 0.355) | 0.0001 |
| DBP | 0.132 (0.049, 0.215) | 0.002 |
| GGT | 0.095 (0.072, 0.118) | 0.0001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obeng-Gyasi, E.; Obeng-Gyasi, B. Blood Pressure and Oxidative Stress among U.S. Adults Exposed to Lead in Military Environments—A Preliminary Study. Diseases 2018, 6, 97. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases6040097
Obeng-Gyasi E, Obeng-Gyasi B. Blood Pressure and Oxidative Stress among U.S. Adults Exposed to Lead in Military Environments—A Preliminary Study. Diseases. 2018; 6(4):97. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases6040097
Chicago/Turabian StyleObeng-Gyasi, Emmanuel, and Barnabas Obeng-Gyasi. 2018. "Blood Pressure and Oxidative Stress among U.S. Adults Exposed to Lead in Military Environments—A Preliminary Study" Diseases 6, no. 4: 97. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases6040097





