Synthetic mRNAs; Their Analogue Caps and Contribution to Disease
Abstract
:1. Introduction
2. The mRNA Caps
2.1. The Caps Used in Synthetic mRNA Formations for Vaccination Purposes
| A/The most common natural quanosine methylated mRNA cap modifications and their biological functions | ||
|---|---|---|
| Name of cap modifications and enzymes involved | Structure and use by different species | Site of capping and biological function |
| Cap O: sequential methylation of the first quanosine nucleotide [5,6] RNA TPase GTase guanine-N7 MTase | m7G(5′)ppp(5′)G Universal for all eukaryotic mRNAs Used by most viruses | Nuclear mRNA capping Recruitment of pre-mRNA protein complex for splicing, polyadenylation and nuclear export. Protection from nonsense mediated decay. Efficient nuclear export by cap binding complex (CBC) Cytoplasm Protection from exonuclease cleavage Affix of elF4E-p to assemble the elF4F complex for initiation of translation. Regulation of gene expression by CBC and elF4E-p. |
| Cap N6A: substitution of first transcribed guanosine nucleotide by adenosine methylated at 6N position [20,21,22] Multi component protein complex consisting of catalytic subunit Methyltransferase Like 3 (METTL3) | m7G(5′)ppp(5′)AmpNp 20–50% of m7G(5′)ppp(5′)Xm mRNA caps in Hela cells Common to human and mouse mRNA Used by selected viruses including Influenza A virus (IAV), HCV, HBV, HIV, Simian virus 40 (CV40), and enterovirus 71 | Co-transcriptional modification. Control of mRNA splicing. Depriving decapping activity. Transcription start site (TSS) signaling. Epitranscriptomic gene regulation. Regulation of viral infection and host immune response. Promotion of RNA Decay. Cap independent mRNA translation. |
| Cap 1: Methylation of the +1 ribonucleotide at the 2′O position of the ribose [6] m7G-specific 2′O methyltransferase (2′O MTase) cap methyltrasnferase 1 (CMTR1) | m7G(5′)ppp(5′)Gm Lower and higher eukaryotes (mouse and human) Selected viruses | Eukaryotes: nuclear co-transcriptional modification. Restriction of Cap O dependent initiation of translation “as non self” during cellular evasion of foreign mRNA. Promotion of antiviral response by induction of interferon stimulated gene (ISG) proteins. Type 1 interferon signaling leads to expression of interferon-induced proteins with tetratricopeptide repeats (IFIT). Binding of IFIT 1 to Cap O instead of elF4E-p attenuates mRNA translation and replication of viruses that do not encode their own 2′O Mtase, e.g., SARS-CoV, West Nile virus. Viruses: post transcriptional modification at cytoplasm. Evasion of recognition by the innate immune response of the host and interferon response by viruses using a) host mRNA capping pathways (conventional capping) e.g., herpesviruses and retroviruses or (b) viral (non-conventional) e.g., coronaviruses and paramyxoviruses expressing their own 2′O Mtase as in case of SARS-CoV-2. |
| Cap 2: Methylation of the +2 ribonucleotide at the 2′O position of the ribose [7] CMTR-2 | m7G(5′)ppp(5′)GmNm Higher eukaryotes (human) | Nuclear and cytoplasmic Independent from N7 methylation of guanosine (Cap O) or from methylation of first nucleotide 2′O ribose (Cap 1). Greater affiliation for cap2 methylation in Cap 1 mRNAs. Used for efficient pre-mRNA splicing (small nuclear RNAs). Promotion of Cap 1 dependent mRNA restriction of translation as “non self” against foreign (viral) mRNA. |
| Cap NAD+: 5′ end NAD+ [9] Bacterial RNA polymerase (RNAP) Eukaryotic RNAP II Sensitive to nuclear migration protein nudC (containing NAD-capped RNA hydrolase) Sensitive to DXO degradation proteins in human | NAD(5′)pNp Bacteria Yeasts Human | Nuclear during initiation of transcription, -3- 4-fold increase in mRNA stability in bacteria. Non canonical initiating nucleotide (NCIN)-mediated initiation of transcription. Mammalian cells are equipped with a distinct NAD+ capping mechanism from transcription initiation that does not support cap-dependent translation. Combination of de-NADing proteins in human control the mRNA decay mechanisms and the gene expression. |
| B/The synthetic mRNA capping systems | ||
| Biochemical synthesis pathways | Structure | Biological properties |
| 5′ terminal mRNA modification by vaccinia virus enzymes [14] Guanylyl transferase and S adenosylmethionine: mRNA (guanine-7) methyltransferase | G(5′)ppp(5′)Gp G(5′)ppp(5′)Ap under presence of S adenosylmethionine m7G(5′)ppp(5′)Gmp m7G(5′)ppp(5′)Amp | Post-transcriptional modification No sequence specificity apart from terminal purine. Acting by function of poly(A) as a substrate for all methylation reactions. |
| Anti-reverse cap analogs (ARCA) [17] Biochemical modifications using pyrophosphate bond formation reactions | 7 methyl(3-deoxy) GpppG 7 methyl(3-O-methyl)GpppG 7 methyl(3-O-methyl)GpppG m7G(5′)ppp(5′)G | Inhibit reverse capping of m7GpppGm by bacteriophage polymerases. Increase 2.3-2.6 fold of mRNA translation compared to m7GpppG cap in a rabbit reticulocyte lysate system Increase protein translation in a dose dependant manner in EL4 cancer cells In combination with optimum poly(A) length and β-globin 3′utranslated regions significantly improve RNA stability in immature dendritic cells (human). |
| Capping enzyme system and 2′O-methyltrasferase to generate Cap 1 [20] | 7-methylGpppGm | Contribution to enhancement of transgene expression in stimulated T cells (human) in combination with optimized UTRs and poly(A) lengths. Equivalent in translation efficiency to the ARCA capping system. |
| Imidodiphosphate: NH analogues, [18] Methylenebisphosphonate:CH2 analogues, [18] Dihalogenmethylenebisphosphonate: CCl2 and CF2 analogues, [18] Biochemical moiety substitution within the 5’,5’-tri- or tetraphosphate bridge of mRNA caps (including ARCAs) | …5′pNHp5′… …5′pCH2p5′… …5′pCl2p5′… …5′pCF2p5′… | Sequential increase in binding affinity to the elF4E Decrease degradation by DcpS (decapping scavenger in exosome) and decapping complex Dcp1–Dcp2 |
| Cap analogues with 1,2-dithiodiphosphate moieties [19] Biochemical dithiodiphosphate modified nucleotide synthesis applied to ARCA synthesis 2S analogs: phosphorothioate (O-to-S) substitution inside the triphosphate bridge 2S ARCA analogs: modified by the presence of a 2′-O-methyl group | 7,2′-O-dimethylGppSpGD1 7,2′-O-dimethylGppspsGD1D2 7,2′-O-dimethylGpppspsGD1D2 | Dramatic increase in ElF4E-p binding affinity Decrease of decapping susceptibility by SpDcp1/2 enzyme complex Increasing efficiency of mRNA translation in immature dendritic cells (D1 to D1D2) D1: gold standard for the treatment of melanoma |
2.2. The mRNA Capping during Normal and Oncogenic Conditions
2.3. The elF4E Dependence Can Be a Provocation to Commencement of Disease
2.4. The elF4E Serine 209 Phosphorylation Is Important for Disease Onset
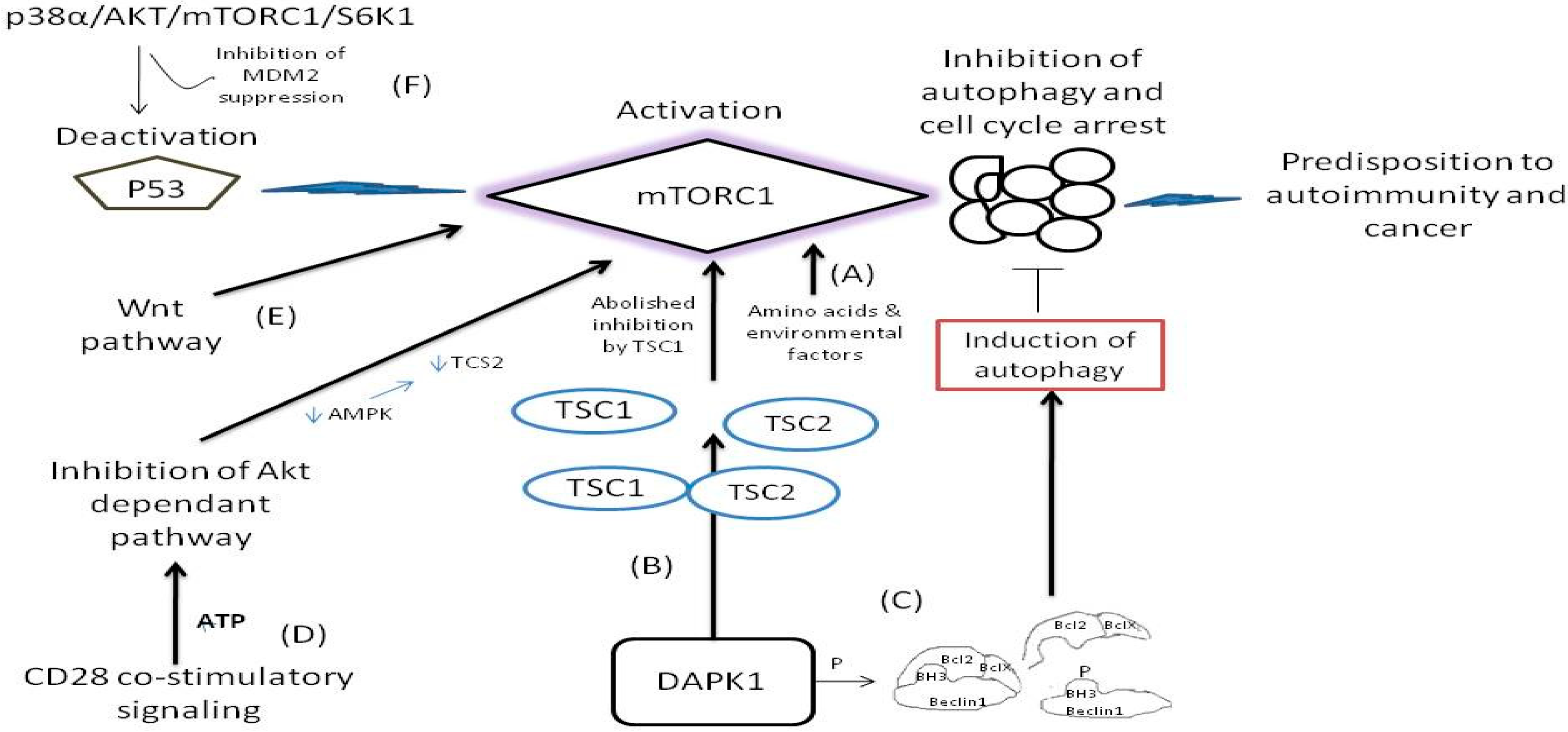
3. The Loss of mTOR Functions
3.1. The Origin of mTOR and Its Links to Disease
3.2. Hamartomas and mTOR Deregulation
3.3. The mTOR and the Immune System Regulation
3.4. The Wnt Signaling Pathway, the mTOR, and the Immune Regulation
3.5. Other Clinically Important Implications Arising by mTOR Improper Signaling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tiezzi, E.B.P.; Pulselli, R.M.; Marcettini, M.; Tiezzi, E. Dissipative Structures in Nature and Human Systems. In WIT Transactions on Ecology and the Environment (Electronic ISSN: 1743-3541). 2008. Available online: https://www.witpress.com/elibrary/wit-transactions-on-ecology-and-the-environment (accessed on 11 June 2021).
- Zotin, A.A.; Porkovskii, V.N. The growth and development of living organisms from the thermodynamic point of view. Phys. A Stat. Mech. Its Appl. 2018, 512, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Kiraga-Motoszko, K.; Niedzwiecka, A.; Modrak-Wojcik, A.; Stepinski, J.; Darzynkiewicz, E.; Stolarski, R. Thermodynamics of molecular recognition of mRNA 5’ cap by yeast eukaryotic initiation factor 4E. J. Phys. Chem. B 2011, 115, 8746–8754. [Google Scholar] [CrossRef]
- Niedzwiecka, A.; Darzynkiewicz, E.; Stolarski, R. Thermodynamics of mRNA 5’ cap binding by eukaryotic translation initiation factor eIF4E. Biochemistry 2004, 43, 13305–13317. [Google Scholar] [CrossRef] [PubMed]
- Anand Ramanathan, G.; Brett, R.; Chan, S.H. mRNA capping: Biological functions and applications. Nucleic Acids Res. 2016, 44, 7511–7526. [Google Scholar] [CrossRef]
- Dimitrova, D.G.; Teysset, L.; Carré, C. RNA 2’-O-Methylation (Nm) Modification in Human Diseases. Genes 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, M.; Purta, E.; Kaminska, K.H.; Cymerman, I.A.; Campbell, D.A.; Mittra, B.; Zamudio, J.R.; Sturm, N.R.; Jaworski, J.; Bujnicki, J.M. 2’-O-ribose methylation of cap2 in human: Function and evolution in a horizontally mobile family. Nucleic Acids Res. 2011, 39, 4756–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balagopal, V.; Parker, R. Polysomes, P bodies and stress granules: States and fates of eukaryotic mRNAs. Curr. Opin. Cell Biol. 2009, 3, 403–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, D.V.; Jackson, B.; Trotman, J.B.; Bundschuh, R.; Schoenberg, D.R. Inhibition of cytoplasmic cap methylation identifies 5′ TOP mRNAs as recapping targets and reveals recapping sites downstream of native 5′ ends. Nucleic Acids Res. 2020, 48, 3806–3815. [Google Scholar] [CrossRef]
- Leung, D.W.; Gaya, K.; Amarasinghe, G.K. When your cap matters: Structural insights into self vs non-self recognition of 5′ RNA by immunomodulatory host proteins. Curr. Opin. Struct. Biol. 2016, 36, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, B.; VanBlargan, L.A.; Xu, W.; White, J.P.; Shan, C.; Shi, P.Y.; Zhang, R.; Adhikari, J.; Gross, M.L.; Leung, D.W.; et al. Human IFIT3 Modulates IFIT1 RNA Binding Specificity and Protein Stability. Immunity 2018, 48, 487–499.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.A.; Paoletti, E.; Moss, B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J. Biol. Chem. 1975, 250, 9322–9329. [Google Scholar] [CrossRef]
- Pasquinelli, A.E.; Dahlberg, J.E.; Lund, E. Reverse 5’ caps in RNAs made in vitro by phage RNA polymerases. RNA 1995, 1, 957–967. [Google Scholar]
- Bohnsack, M.T.; Sloan, K.E. Modifications in small nuclear RNAs and their roles in spliceosome assembly and function. Biol. Chem. 2018, 399, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Stepinski, J.; Waddell, C.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Synthesis and properties of mRNAs containing the novel “anti-reverse” cap analogs 7-methyl(3’-O-methyl)GpppG and 7-methyl(3’-deoxy)GpppG. RNA 2001, 7, 1486–1495. [Google Scholar] [PubMed]
- Rydzik, A.M.; Warminski, M.; Sikorski, P.J.; Baranowski, M.R.; Walczak, S.; Kowalska, J.; Zuberek, J.; Lukaszewicz, M.; Nowak, E.W.; Claridge, T.D. mRNA cap analogues substituted in the tetraphosphate chain with CX2: Identification of O-to-CCl2 as the first bridging modification that confers resistance to decapping without impairing translation. Nucleic Acids Res. 2017, 45, 8661–8675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strenkowska, M.; Grzela, R.; Majewski, M.; Wnek, K.; Kowalska, J.; Lukaszewicz, M.; Zuberek, J.; Darzynkiewicz, E.; Kuhn, A.N.; Sahin, U.; et al. Cap analogs modified with 1,2-dithiodiphosphate moiety protect mRNA from decapping and enhance its translational potential. Nucleic Acids Res. 2016, 44, 9578–9590. [Google Scholar] [CrossRef]
- Zhao, Y.; Moon, E.; Carpenito, C.; Paulos, C.M.; Liu, X.; Brennan, A.L.; Chew, A.; Carroll, R.G.; Scholler, J.; Levine, B.L.; et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010, 70, 9053–9061. [Google Scholar] [CrossRef] [Green Version]
- Galloway, A.; Cowling, V.H. mRNA cap regulation in mammalian cell function and fate. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 270–279. [Google Scholar] [CrossRef]
- Lin, X.; Chai, G.; Wu, Y.; Li, J.; Chen, F.; Liu, J.; Luo, G.; Tauler, J.; Du, J.; Lin, S.; et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat. Commun. 2019, 10, 2065. [Google Scholar] [CrossRef]
- Jiao, X.; Doamekpor, S.K.; Bird, J.G.; Nickels, B.E.; Tong, L.; Hart, R.P.; Kiledjian, M. 5’ End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding. Cell 2017, 168, 1015–1027.e10. [Google Scholar] [CrossRef] [Green Version]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, O.; Sahin, U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Uttam, S.; Wong, C.; Price, T.J.; Khoutorsky, A. eIF4E-Dependent Translational Control: A Central Mechanism for Regulation of Pain Plasticity. Front. Genet. 2018, 9, 470. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Truitt, M.L.; Conn, C.S.; Shi, Z.; Pang, X.; Tokuyasu, T.; Coady, A.M.; Seo, Y.; Barna, M.; Ruggero, D. Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell 2015, 162, 59–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.D.; Cowling, V.H. Specific regulation of mRNA cap methylation by the c-Myc and E2F1 transcription factors. Oncogene 2009, 28, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.J.; Xie, D. RACK1, a versatile hub in cancer. Oncogene. 2015, 34, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic, B.; Topisirovic, I.; Borden, K.L. Controlling gene expression through RNA regulons: The role of the eukaryotic translation initiation factor eIF4E. Cell Cycle 2007, 6, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Xu, X.S.; Russell, J.E. A nucleolin-binding 3’ untranslated region element stabilizes beta-globin mRNA in vivo. Mol. Cell. Biol. 2006, 26, 2419–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Sanchez, M.E.; Gonatopoulos-Pournatzis, T.; Preston, G.; Lawlor, M.A.; Cowling, V.H. S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation. Mol. Cell. Biol. 2009, 29, 6182–6191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graff, J.R.; Zimmer, S.G. Translational control and metastatic progression: Enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin. Exp. Metastasis 2003, 20, 265–273. [Google Scholar] [CrossRef]
- Martineau, Y.; Azar, R.; Bousquet, C.; Pyronnet, S. Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene 2013, 32, 671–677. [Google Scholar] [CrossRef]
- Mochizuki, K.; Oguro, A.; Ohtsu, T.; Sonenberg, N.; Nakamura, Y. High affinity RNA for mammalian initiation factor 4E interferes with mRNA-cap binding and inhibits translation. RNA 2005, 11, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Horton, L.E.; Bushell, M.; Barth-Baus, D.; Tilleray, V.J.; Clemens, M.J.; Hensold, J.O. p53 activation results in rapid dephosphorylation of the eIF4E-binding protein 4E-BP1, inhibition of ribosomal protein S6 kinase and inhibition of translation initiation. Oncogene 2002, 21, 5325–5334. [Google Scholar] [CrossRef] [Green Version]
- Yi, W.; Gupta, S.; Ricker, E.; Manni, M.; Jessberger, R.; Chinenov, Y.; Molina, H.; Pernis, A.B. The mTORC1-4E-BP-eIF4E axis controls de novo Bcl6 protein synthesis in T cells and systemic autoimmunity. Nat. Commun. 2017, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- She, Q.B.; Halilovic, E.; Ye, Q.; Zhen, W.; Shirasawa, S.; Sasazuki, T.; Solit, D.B.; Rosen, N. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010, 18, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, H.; Li, X.; Xu, X.; Leibowitz, B.J.; Tong, J.; Chen, L.; Ao, L.; Xing, W.; Luo, J.; Yu, Y.; et al. eIF4E S209 phosphorylation licenses myc- and stress-driven oncogenesis. eLife 2020, 9, e60151. [Google Scholar] [CrossRef] [PubMed]
- Ilic, N.; Utermark, T.; Widlund, H.R.; Roberts, T.M. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc. Natl. Acad. Sci. USA 2011, 108, E699–E708. [Google Scholar] [CrossRef] [Green Version]
- Kerins, M.J.; Ooi, A. The Roles of NRF2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018, 29, 1756–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, J.G.; Zhang, Y.; Tian, Y.; Panova, N.; Barvík, I.; Greene, L.; Liu, M.; Buckley, B.; Krásný, L.; Lee, J.K.; et al. The mechanism of RNA 5′ capping with NAD+, NADH and desphospho-CoA. Nature 2016, 535, 444–447. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Radiskym, E.; Leibold, E.A. The iron-responsive element binding protein. Purification, cloning, and regulation in rat liver. J. Biol. Chem. 1992, 267, 19005–19010. [Google Scholar] [CrossRef]
- Mignone, F.; Gissi, C.; Liuni, S.; Pesole, G. Untranslated regions of mRNAs. Genome Biol. 2002, 3, REVIEWS0004. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Inoki, K.; Corradetti, M.N.; Guan, K.L. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005, 37, 19–24. [Google Scholar] [CrossRef]
- Dazert, E.; Hall, M.N. mTOR signaling in disease. Curr. Opin. Cell Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachdi, L.; Balcazar, N.; Osorio-Duque, F.; Elghazi, L.; Weiss, A.; Gould, A.; Chang-Chen, K.J.; Gambello, M.J.; Bernal-Mizrachi, E. Disruption of Tsc2 in pancreatic beta cells induces beta cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc. Natl. Acad. Sci. USA 2008, 105, 9250–9255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shigeyama, Y.; Kobayashi, T.; Kido, Y.; Hashimoto, N.; Asahara, S.; Matsuda, T.; Takeda, A.; Inoue, T.; Shibutani, Y.; Koyanagi, M.; et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol. Cell Biol. 2008, 28, 2971–2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elghazi, L.; Balcazar, N.; Blandino-Rosano, M.; Cras-Méneur, C.; Fatrai, S.; Gould, A.P.; Chi, M.M.; Moley, K.H.; Bernal-Mizrachi, E. Decreased IRS signaling impairs beta-cell cycle progression and survival in transgenic mice overexpressing S6K in beta-cells. Diabetes 2010, 59, 2390–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avruch, J.; Belham, C.; Weng, Q.; Hara, K.; Yonezawa, K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol. 2001, 26, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Meyuhas, O. Synthesis of the translational apparatus is regulated at the translational level. Eur. J. Biochem. 2000, 267, 6321–6330. [Google Scholar] [CrossRef]
- Hasty, P.; Sharp, Z.D.; Curiel, T.J.; Campisi, J. mTORC1 and p53: Clash of the gods? Cell Cycle 2013, 12, 20–25. [Google Scholar] [CrossRef]
- Gingras, A.C.; Raught, B.; Sonenberg, N. eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999, 68, 913–963. [Google Scholar] [CrossRef]
- Harris, T.E.; Lawrence, J.C., Jr. TOR signaling. Sci. STKE 2003, 2003, re15. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Hall, M.N. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 2003, 4, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Singh, P.; Ravanan, P.; Talwar, P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Zalckvar, E.; Berissi, H.; Eisenstein, M.; Kimchi, A. Phosphorylation of Beclin 1 by DAP-kinase promotes autophagy by weakening its interactions with Bcl-2 and Bcl-XL. Autophagy 2009, 5, 720–722. [Google Scholar] [CrossRef] [Green Version]
- Soni, A.; Akcakanat, A.; Singh, G.; Luyimbazi, D.; Zheng, Y.; Kim, D.; Gonzalez-Angulo, A.; Meric-Bernstam, F. eIF4E knockdown decreases breast cancer cell growth without activating Akt signaling. Mol. Cancer Ther. 2008, 7, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- El-Hashemite, N.; Walker, V.; Zhang, H.; Kwiatkowski, D.J. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 2003, 63, 5173–5177. [Google Scholar]
- Wu, Y.; Zhou, B.P. Kinases meet at TSC. Cell Res. 2007, 17, 971–973. [Google Scholar] [CrossRef] [Green Version]
- Varin, J.; Poulain, L.; Hivelin, M.; Nusbaum, P.; Hubas, A.; Laurendeau, I.; Lantieri, L.; Wolkenstein, P.; Vidaud, M.; Pasmant, E.; et al. Dual mTORC1/2 inhibition induces anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumor cells. Oncotarget 2016, 7, 35753–35767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The ABC of APC. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Powell, J.D.; Pollizzi, K.N.; Heikamp, E.B.; Horton, M.R. Regulation of Immune Responses by mTOR. Annu. Rev. Immunol. 2012, 30, 39–68. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto., K.; Makino, Y.; Pereira, T.; Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000, 19, 4298–4309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddur, M.S.; Miossec, P.; Kaveri, S.V.; Bayry, J. Th17 Cells: Biology, Pathogenesis of Autoimmune and Inflammatory Diseases, and Therapeutic Strategies. Am. J. Pathol 2012, 181, 8–18. [Google Scholar] [CrossRef]
- Yuko, K.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Benchabane, H.; Ahmed, Y. The adenomatous polyposis coli tumor suppressor and Wnt signaling in the regulation of apoptosis. Adv. Exp. Med. Biol. 2009, 656, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Prossomariti, A.; Piazzi, G.; Alquati, C.; Ricciardiello, L. Are Wnt/β-Catenin and PI3K/AKT/mTORC1 Distinct Pathways in Colorectal Cancer? Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 491–506. [Google Scholar] [CrossRef]
- Bour-Jordan, H.; Blueston, J.A. CD28 function: A balance of costimulatory and regulatory signals. J. Clin. Immunol. 2002, 22, 1–7. [Google Scholar] [CrossRef]
- Brosens, L.A.; Langeveld, D.; van Hattem, W.A.; Giardiello, F.M.; Offerhaus, G.J. Juvenile polyposis syndrome. World J. Gastroenterol. 2011, 17, 4839–4844. [Google Scholar] [CrossRef]
- Karner, C.M.; Lee, S.Y.; Long, F. Bmp Induces Osteoblast Differentiation through both Smad4 and mTORC1 Signaling. Mol. Cell Biol. 2017, 37, e00253-16. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, G.M.; Dennis, P.A. PTEN hamartoma tumor syndromes. Eur. J. Hum. Genet. 2008, 16, 1289–1300. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Papadopoulos, E.; Hagner, P.R.; Wagner, G. Hypoxia-inducible factor-1α (HIF-1α) promotes cap-dependent translation of selective mRNAs through up-regulating initiation factor eIF4E1 in breast cancer cells under hypoxia conditions. J. Biol. Chem. 2013, 288, 18732–18742. [Google Scholar] [CrossRef] [Green Version]
- Waickman, A.T.; Powell, J.D. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 2012, 249, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.H.; Liu, L.Z. Role of mTOR in anticancer drug resistance: Perspectives for improved drug treatment. Drug Resist. Updates 2008, 11, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Pollizzim, K.N.; Sun, I.H.; Patel, C.H.; Lo, Y.C.; Oh, M.H.; Waickman, A.T.; Tam, A.J.; Blosser, R.L.; Wen, J.; Delgoffe, G.M.; et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8+ T cell differentiation. Nat. Immunol. 2016, 17, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Verbist, K.C.; Guy, C.S.; Milasta, S.; Liedmann, S.; Kamiński, M.M.; Wang, R.; Green, D.R. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 2016, 532, 389–393. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Spangle, J.M.; Ohlson, C.E.; Cheng, H.; Roberts, T.M.; Cantley, L.C.; Zhao, J.J. PI3K-p110α mediates the oncogenic activity induced by loss of the novel tumor suppressor PI3K-p85α. Proc. Natl. Acad. Sci. USA 2017, 114, 7095–7100. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu. Rev. Pathol. 2013, 8, 477–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leprivier, G.; Rotblat, B. How does mTOR sense glucose starvation? AMPK is the usual suspect. Cell Death Discov. 2020, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Kapahi, P.; Chen, D.; Rogers, A.N.; Katewa, S.D.; Li, P.W.; Thomas, E.L.; Kockel, L. With TOR, less is more: A key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010, 11, 453–465. [Google Scholar] [CrossRef] [Green Version]
- Castilho, R.M.; Squarize, C.H.; Chodosh, L.A.; Williams, B.O.; Gutkind, J.S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 2009, 5, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.R. The Wnts. Genome Biol. 2002, 3, REVIEWS3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, A.Y.; Roux, P.P.; Blenis, J. Mind the GAP: Wnt steps onto the mTORC1 train. Cell 2006, 126, 834–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, O.H.; Valdez, R.; Theisen, B.K.; Guo, W.; Ferguson, D.O.; Wu, H.; Morrison, S.J. Pten dependence distinguishes hematopoietic stem cells from leukemia-initiating cells. Nature 2006, 441, 475–482. [Google Scholar] [CrossRef] [Green Version]
- Mondino, A.; Mueller, D.L. mTOR at the crossroads of T cell proliferation and tolerance. Semin. Immunol. 2007, 19, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Lipton, J.O.; Sahinm, M. The neurology of mTOR. Neuron 2014, 84, 275–791. [Google Scholar] [CrossRef] [Green Version]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyamam, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Liu, Y.; Zheng, P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2009, 2, ra75. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Benign Tumors | Ways of Sensitivity to elF4E |
|---|---|
| Hamartomas | PTEN/P13K/AKT pathways [46,76] |
| Benign disorders that lead to malignancy | |
| Neurofibromatosis type 1 | AKT/Raf/MEK/ERK pathways [65,66,67] |
| Von Hippel-Lindau syndrome | Hypoxia inducible factor 1 [66,67,77] |
| Family adenomatous polyposis | Wnt/β-catenin signaling P13K/AKT [68] |
| Juvenile polyposis syndrome | Smad 4 pathway [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakopoulos, A.M.; McCullough, P.A. Synthetic mRNAs; Their Analogue Caps and Contribution to Disease. Diseases 2021, 9, 57. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases9030057
Kyriakopoulos AM, McCullough PA. Synthetic mRNAs; Their Analogue Caps and Contribution to Disease. Diseases. 2021; 9(3):57. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases9030057
Chicago/Turabian StyleKyriakopoulos, Anthony M., and Peter A. McCullough. 2021. "Synthetic mRNAs; Their Analogue Caps and Contribution to Disease" Diseases 9, no. 3: 57. https://0-doi-org.brum.beds.ac.uk/10.3390/diseases9030057








