Preliminary Protocol Development of a HPLC-TBARS-EVSC (Ex Vivo Stratum Corneum) Assay for Skin Research: Application in a Sunscreen System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Prototype Formulation
2.3. Instrumentation
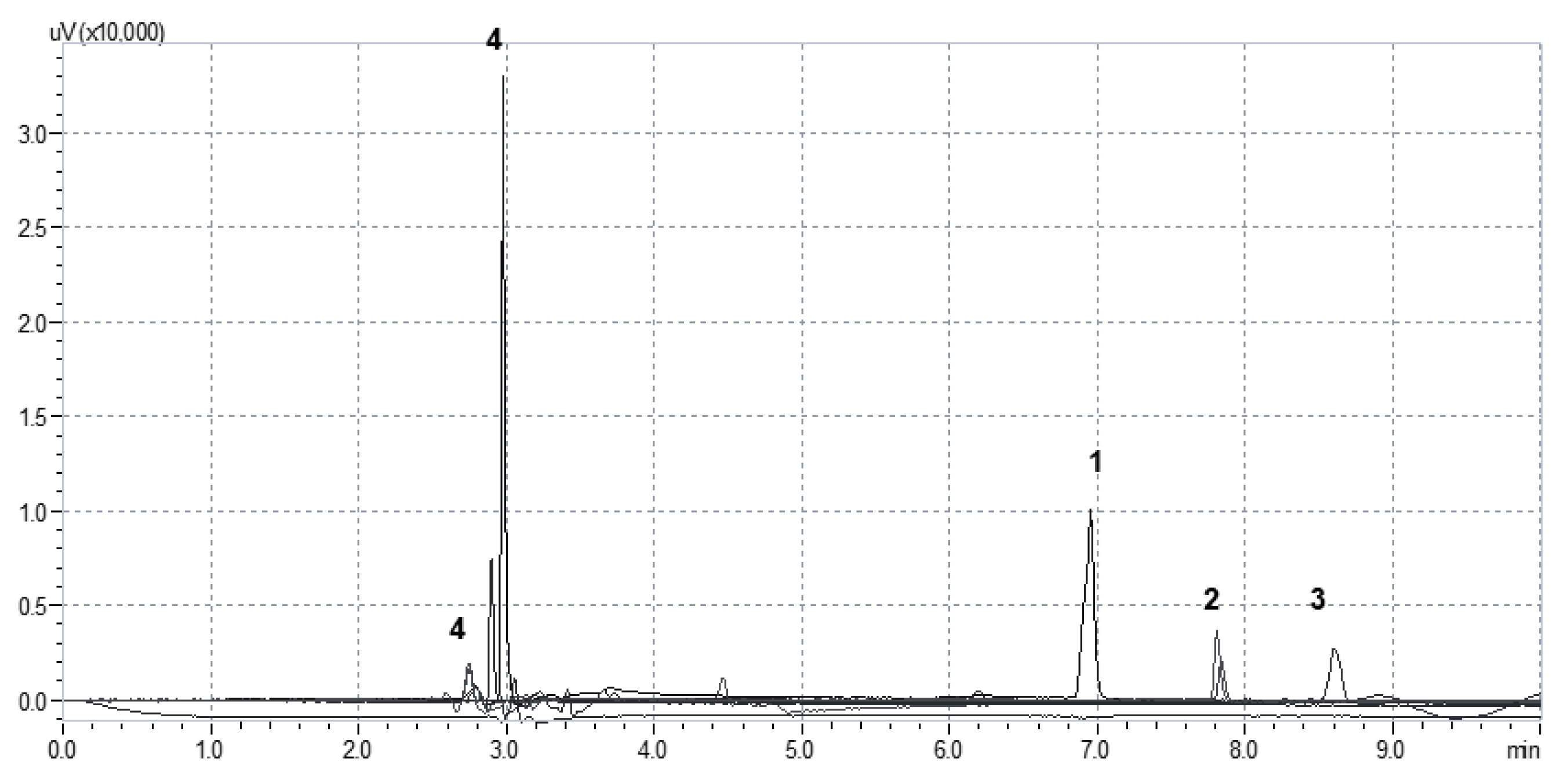
2.4. Determination of Stratum Corneum Lipid Peroxidation by TBARS-HPLC Assay
2.5. MDA Stock Solution
2.6. Preparation of the Reaction
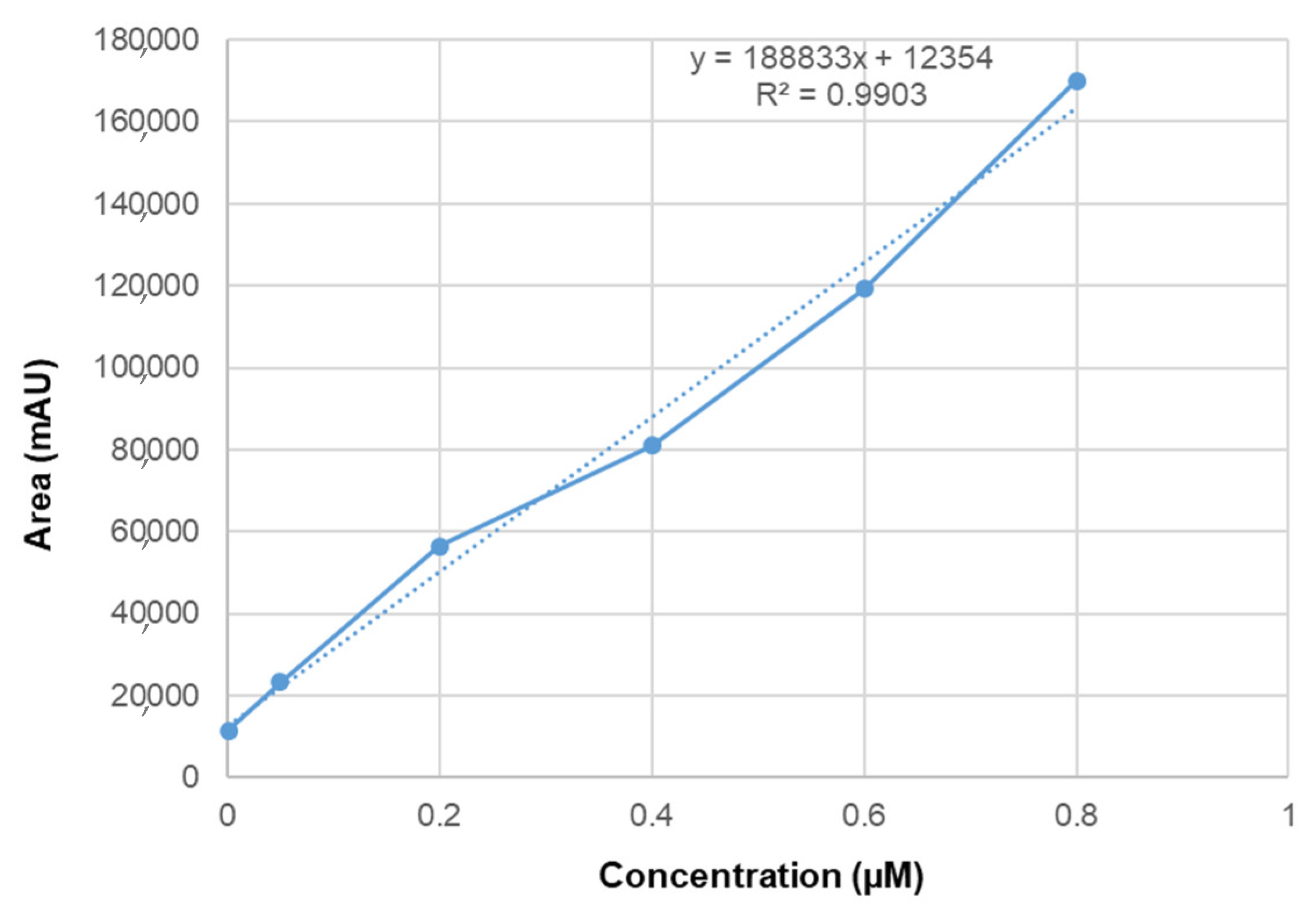
2.7. Linearity and Selectivity
2.8. Ethical Issue
2.9. Ex Vivo Protocol and Stratum Corneum Sample
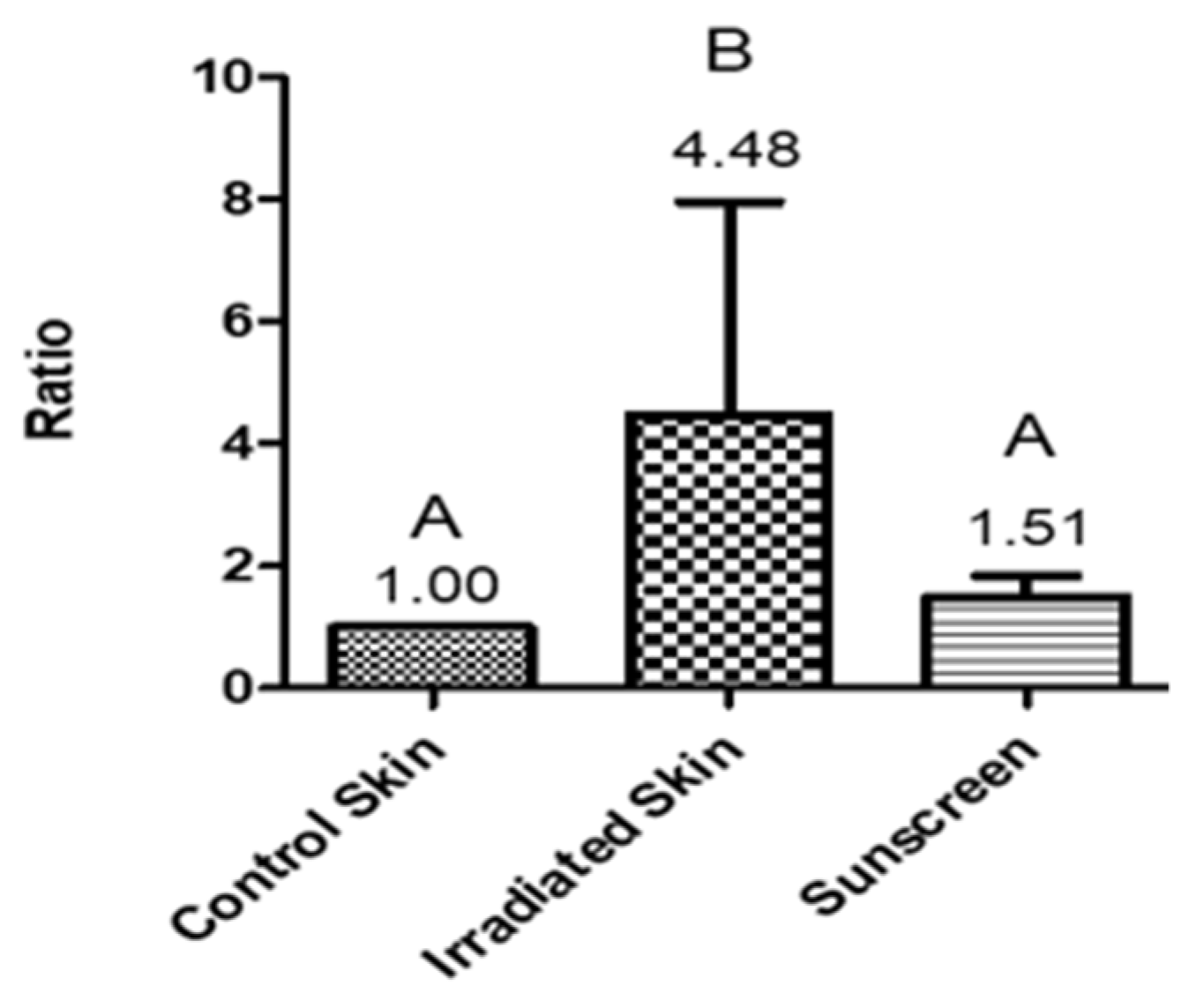
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.P.; Chandra, A.; Mahdi, F.; Roy, A.; Sharma, P. Reconvene and reconnect the antioxidant hypothesis in human health and disease. Indian J. Clin. Biochem. 2010, 25, 225–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A. A current view on inflammation. Nat. Immunol. 2017, 18, 825. [Google Scholar] [CrossRef] [Green Version]
- Walsh, L.J. Mast cells and oral inflammation. Crit. Rev. Oral Biol. Med. 2003, 14, 188–198. [Google Scholar] [CrossRef]
- Peres, D.D.A.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Sauce, R.; Pinto, C.A.S.D.O.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Ex vivo penetration analysis and anti-inflammatory efficacy of the association of ferulic acid and UV filters. Eur. J. Pharm. Sci. 2021, 156. [Google Scholar] [CrossRef]
- Potard, G.; Laugel, C.; Baillet, A.; Schaefer, H.; Marty, J.P. Quantitative HPLC analysis of sunscreens and caffeine during in vitro percutaneous penetration studies. Int. J. Pharm. 1999, 189, 249–260. [Google Scholar] [CrossRef]
- Bastos, A.S.; Loureiro, A.P.D.M.; De Oliveira, T.F.; Corbi, S.C.T.; Caminaga, R.M.S.; Rossa, C.; Orrico, S.R.P. Quantitation of malondialdehyde in gingival crevicular fluid by a high-performance liquid chromatography-based method. Anal. Biochem. 2012, 423, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.L.; Yeh, S.L.; Chang, C.Y.; Hu, M.L. Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clin. Biochem. 2000, 33, 619–625. [Google Scholar] [CrossRef]
- Benfeldt, E.; Hansen, S.H.; Vølund, A.; Menné, T.; Shah, V.P. Bioequivalence of topical formulations in humans: Evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J. Investig. Dermatol. 2007, 127, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, C.; Barba, C.; Rubio, L.; Scott, S.; Kilimnik, A.; Coderch, L.; Notario, J.; Parra, J.L. An ex vivo methodology to assess the lipid peroxidation in stratum corneum. J. Photochem. Photobiol. B. 2009, 97, 71–76. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; Peres, D.D.; Rugno, C.M.; Kojima, M.; de Pinto, C.A.S.O.; Consiglieri, V.O.; Kaneko, T.M.; Rosado, C.; Mota, J.; Velasco, M.V.R.; et al. Functional photostability and cutaneous compatibility of bioactive UVA sun care products. J. Photochem. Photobiol. B Biol. 2015, 148, 154–159. [Google Scholar] [CrossRef]
- Peres, D.D. Ácido Ferúlico em Protetores Solares: Desenvolvimento e Eficácia Multifuncional in vitro, ex vivo e in vivo. Ph.D. Thesis, University of São Paulo, Sao Paulo, Brazil, 2015. [Google Scholar]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Cândido, T.M.; de Oliveira, C.A.; Ariede, M.B.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and Antioxidant Efficacy Profiles of Rutin-Loaded Ethosomes for Topical Application. AAPS PharmSciTech 2018. [Google Scholar] [CrossRef]
- Ich ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Int. Conf. Harmon. 2005, 1994, 17.
- Devasagayam, T.P.A.; Boloor, K.K.; Ramasarma, T. Methods for estimating lipid peroxidation: An analysis of merits and demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar]
- Yagi, K.; Nishigaki, I.; Ohama, H. Measurement of serum TBA value. Vitamin 1968, 37, 105–112. [Google Scholar]
- Alessio, H. Handbook of Oxidants and Antioxidants in Exercise; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Kosugi, H.; Kikugawa, K. Thiobarbituric acid reaction of aldehydes and oxidized lipids in glacial acetic acid. Lipids 1985, 20, 915–921. [Google Scholar] [CrossRef]
- Buttkus, H.; Bose, R.J. Amine-malonaldehyde condensation products and their relative color contribution in the thiobarbituric acid test. J. Am. Oil Chem. Soc. 1972, 49, 440–443. [Google Scholar] [CrossRef]
- Moselhy, H.F.; Reid, R.G.; Yousef, S.; Boyle, S.P. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J. Lipid Res. 2013, 54, 852–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dib, M.; Garrel, C.; Favier, A.; Robin, V.; Desnuelle, C. Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J. Neurol. 2002, 249, 367–374. [Google Scholar] [CrossRef]
- Panjamurthy, K.; Manoharan, S.; Ramachandran, C.R. Lipid peroxidation and antioxidant status in patients with periodontitis. Cell. Mol. Biol. Lett. 2005, 10, 255–264. [Google Scholar] [PubMed]
- Yagi, K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem. Med. 1976, 15, 212–216. [Google Scholar] [CrossRef]
- Jardine, D.; Antolovich, M.; Prenzler, P.D.; Robards, K. Liquid chromatography-mass spectrometry (LC-MS) investigation of the thiobarbituric acid reactive substances (TBARS) reaction. J. Agric. Food Chem. 2002, 50, 1720–1724. [Google Scholar] [CrossRef]
- Knight, J.A.; Robert, K.; Mcclellan, L. Specificity of the thiobarbituric acid reaction: Its use in studies of lipid peroxidation. Clin. Chem. 1988, 34, 2433–2438. [Google Scholar] [CrossRef]
- Abuja, P.M.; Albertini, R. Methods for monitoring oxidative stress, lipid peroxidation and oxidation resistance of lipoproteins. Clin. Chim. Acta 2001, 306, 1–17. [Google Scholar] [CrossRef]
- Al-Rimawi, F. Development and Validation of a Simple Reversed-Phase HPLC-UV Method for Determination of Malondialdehyde in Olive Oil. J. Am. Oil Chem. Soc. 2015, 92, 933–937. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; Dario, M.F.; Sarruf, F.D.; Mariz, I.F.A.; Velasco, M.V.R.; Rosado, C.; Baby, A.R. Safety and efficacy evaluation of gelatin-based nanoparticles associated with UV filters. Colloids Surf. B Biointerfaces 2015, 140, 531–537. [Google Scholar] [CrossRef]
- Graziola, F.; Candido, T.M.; De Oliveira, C.A.; Peres, D.D.A.; Georges Issa, M.; Mota, J.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; et al. Gelatin-based microspheres crosslinked with glutaraldehyde and rutin oriented to cosmetics. Artic. Braz. J. Pharm. Sci. 2016, 52, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Peres, D.A.; de Oliveira, C.A.; da Costa, M.S.; Tokunaga, V.K.; Mota, J.P.; Rosado, C.; Consiglieri, V.O.; Kaneko, T.M.; Velasco, M.V.R.; Baby, A.R. Rutin increases critical wavelength of systems containing a single UV filter and with good skin compatibility. Ski. Res. Technol. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Tuchinda, C.; Lim, H.W.; Osterwalder, U.; Rougier, A. Novel Emerging Sunscreen Technologies. Dermatol. Clin. 2006, 24, 105–117. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauce, R.; Pinto, C.A.S.d.O.; Ayala-Jara, C.; Prieto, Z.A.; Velasco, M.V.R.; Baby, A.R. Preliminary Protocol Development of a HPLC-TBARS-EVSC (Ex Vivo Stratum Corneum) Assay for Skin Research: Application in a Sunscreen System. Sci. Pharm. 2021, 89, 17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm89020017
Sauce R, Pinto CASdO, Ayala-Jara C, Prieto ZA, Velasco MVR, Baby AR. Preliminary Protocol Development of a HPLC-TBARS-EVSC (Ex Vivo Stratum Corneum) Assay for Skin Research: Application in a Sunscreen System. Scientia Pharmaceutica. 2021; 89(2):17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm89020017
Chicago/Turabian StyleSauce, Rafael, Claudinéia Aparecida Sales de Oliveira Pinto, Carmen Ayala-Jara, Zulita Adriana Prieto, Maria Valéria Robles Velasco, and André Rolim Baby. 2021. "Preliminary Protocol Development of a HPLC-TBARS-EVSC (Ex Vivo Stratum Corneum) Assay for Skin Research: Application in a Sunscreen System" Scientia Pharmaceutica 89, no. 2: 17. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm89020017






