Amphiphilic Alkylated Pectin Hydrogels for Enhanced Topical Delivery of Fusidic Acid: Formulation and In Vitro Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
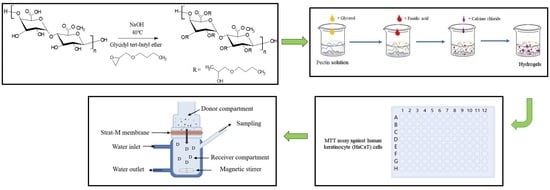
2.2. Synthesis of Alkylated Pectin Derivative
2.3. Characterization of Alkylated Pectin Derivative
- DA (%): degree of alkylation (the alkyl chains number connected to 100 pectin molecules),
- A: the alkyl chain end CH3 integral (δ 1.1 ppm), and
- B: the glucopyranosic ring anomeric proton integral (δ 5.0 ppm).
2.4. Fabrication of Fusidic Acid-Loaded Hydrogels
2.5. Swelling Studies
2.6. Drug Loading and Release Studies
2.7. Cytotoxicity Assay
2.8. In Vitro Permeability Studies
2.9. Data Statistical Analysis
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercuri, M.; Fernandez Rivas, D. Challenges and opportunities for small volumes delivery into the skin. Biomicrofluidics 2021, 15, 11301. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Murnane, K.S.; Blough, B.E.; Banga, A.K. Effects of chemical and physical enhancement techniques on transdermal delivery of 3-fluoroamphetamine hydrochloride. Int. J. Pharm. 2017, 528, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Talukder, M.M.U. Chemical enhancer: A simplistic way to modulate barrier function of the stratum corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Godtfredsen, W.O.; Jahnsen, S.; Lorck, H.; Roholt, K.; Tybring, L. Fusidic acid: A new antibiotic. Nature 1962, 193, 987. [Google Scholar] [CrossRef]
- Eid, A.M.; Istateyeh, I.; Salhi, N.; Istateyeh, T. Antibacterial activity of Fusidic acid and sodium Fusidate nanoparticles incorporated in pine oil Nanoemulgel. Int. J. Nanomed. 2019, 14, 9411. [Google Scholar] [CrossRef] [Green Version]
- Neves, S.C.; Gomes, D.B.; Sousa, A.; Bidarra, S.J.; Petrini, P.; Moroni, L.; Barrias, C.C.; Granja, P.L. Biofunctionalized pectin hydrogels as 3D cellular microenvironments. J. Mater. Chem. B 2015, 3, 2096–2108. [Google Scholar] [CrossRef] [Green Version]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Lal, A.M.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Arafat, M.; Sarfraz, M.; Górecki, D.C.; Barbu, E. Butylglyceryl pectin nanoparticles: Synthesis, formulation and characterization. Polymers 2019, 11, 789. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zheng, C.; Huang, B.; Fei, P. Preparation of acylated pectin with gallic acid through enzymatic method and their emulsifying properties, antioxidation activities and antibacterial activities. Int. J. Biol. Macromol. 2020, 165, 198–204. [Google Scholar] [CrossRef]
- Kocaaga, B.; Kurkcuoglu, O.; Tatlier, M.; Batirel, S.; Guner, F.S. Low-methoxyl pectin–zeolite hydrogels controlling drug release promote in vitro wound healing. J. Appl. Polym. Sci. 2019, 136, 47640. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Meng, Y.; Li, J.; Li, J. An injectable, self-healing hydrogel system from oxidized pectin/chitosan/γ-Fe2O3. Int. J. Biol. Macromol. 2020, 164, 4566–4574. [Google Scholar] [CrossRef]
- Patil, S.B.; Inamdar, S.Z.; Reddy, K.R.; Raghu, A.V.; Akamanchi, K.G.; Inamadar, A.C.; Das, K.K.; Kulkarni, R.V. Functionally tailored electro-sensitive poly (acrylamide)-g-pectin copolymer hydrogel for transdermal drug delivery application: Synthesis, characterization, in-vitro and ex-vivo evaluation. Drug Deliv. Lett. 2020, 10, 185–196. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef]
- Oveissi, F.; Tavakoli, N.; Minaiyan, M.; Mofid, M.R.; Taheri, A. Alginate hydrogel enriched with Ambystoma mexicanum epidermal lipoxygenase-loaded pectin nanoparticles for enhanced wound healing. J. Biomater. Appl. 2020, 34, 1171–1187. [Google Scholar] [CrossRef]
- Chang, H.; Li, C.; Huang, R.; Su, R.; Qi, W.; He, Z. Amphiphilic hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 2899–2910. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Barbu, E.; Liew, K. Bin Hydrophobically Grafted Pullulan Nanocarriers for Percutaneous Delivery: Preparation and Preliminary In Vitro Characterisation. Polymers 2021, 13, 2852. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef] [Green Version]
- Aditya, A.; Chattopadhyay, S.; Gupta, N.; Alam, S.; Veedu, A.P.; Pal, M.; Singh, A.; Santhiya, D.; Ansari, K.M.; Ganguli, M. ZnO nanoparticles modified with an amphipathic peptide show improved photoprotection in skin. ACS Appl. Mater. Interfaces 2018, 11, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-C.; Zheng, H.-L.; Wang, X.-R.; Zheng, X.-L.; Chen, Y.; Fei, W.-D.; Zheng, Y.-Q.; Wang, W.-X.; Zheng, C.-H. Enhanced percutaneous delivery of methotrexate using micelles prepared with novel cationic amphipathic material. Int. J. Nanomed. 2020, 15, 3539. [Google Scholar] [CrossRef] [PubMed]
- Rodoplu, S.; Celik, B.E.; Kocaaga, B.; Ozturk, C.; Batirel, S.; Turan, D.; Guner, F.S. Dual effect of procaine-loaded pectin hydrogels: Pain management and in vitro wound healing. Polym. Bull. 2021, 78, 2227–2250. [Google Scholar] [CrossRef]
- Fan, L.; Cao, M.; Gao, S.; Wang, W.; Peng, K.; Tan, C.; Wen, F.; Tao, S.; Xie, W. Preparation and characterization of a quaternary ammonium derivative of pectin. Carbohydr. Polym. 2012, 88, 707–712. [Google Scholar] [CrossRef]
- Arafat, M.; Fahelelbom, K.M.; Sarfraz, M.K.; Bostanudin, M.F.; Sharif, Q.-A.; Esmaeil, A.; Hanbali, O.A.A.L.; Aburuz, S. Comparison between branded and generic furosemide 40 mg tablets using thermal gravimetric analysis and Fourier transform infrared spectroscopy. J. Pharm. Bioallied Sci. 2020, 12, 489. [Google Scholar] [CrossRef]
- Demir, D.; Ceylan, S.; Göktürk, D.; Bölgen, N. Extraction of pectin from albedo of lemon peels for preparation of tissue engineering scaffolds. Polym. Bull. 2021, 78, 2211–2226. [Google Scholar] [CrossRef]
- Panda, H. Epoxy Resins Technology Handbook (Manufacturing Process, Synthesis, Epoxy Resin Adhesives and Epoxy Coatings); Asia Pacific Business Press Inc.: New Delhi, India, 2019; ISBN 8178331829. [Google Scholar]
- Bostanudin, M.F.; Muhamad Noor, N.S.; Sahudin, S.; Mat Lazim, A.; Tan, S.F.; Sarker, M.Z.I. Investigations of amphiphilic butylglyceryl-functionalized dextran nanoparticles for topical delivery. J. Appl. Polym. Sci. 2021, 138, 50235. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Salam, A.; Mahmood, A.; Arafat, M.; Kaharudin, A.N.; Sahudin, S.; Lazim, A.M.; Azfaralariff, A. Formulation and In-vitro Characterisation of Cross-linked Amphiphilic Guar Gum Nanocarriers for Percutaneous Delivery of Arbutin. J. Pharm. Sci. 2021, 110, 3907–3918. [Google Scholar] [CrossRef]
- Kazemi, M.; Khodaiyan, F.; Hosseini, S.S. Utilization of food processing wastes of eggplant as a high potential pectin source and characterization of extracted pectin. Food Chem. 2019, 294, 339–346. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Toman, P.; Lien, C.-F.; Ahmad, Z.; Dietrich, S.; Smith, J.R.; An, Q.; Molnár, É.; Pilkington, G.J.; Górecki, D.C.; Tsibouklis, J. Nanoparticles of alkylglyceryl-dextran-graft-poly (lactic acid) for drug delivery to the brain: Preparation and in vitro investigation. Acta Biomater. 2015, 23, 250–262. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41, 101–106. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Richel, A.; Blecker, C.; Boufi, S.; Attia, H.; Garna, H. Effects of extraction procedures and plasticizer concentration on the optical, thermal, structural and antioxidant properties of novel ulvan films. Int. J. Biol. Macromol. 2019, 135, 647–658. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Liu, Y.; Wang, Y.; Li, H.; Yu, J. The use of of inulin, maltitol and lecithin as fat replacers and plasticizers in a model reduced-fat mozzarella cheese-like product. J. Sci. Food Agric. 2019, 99, 5586–5593. [Google Scholar] [CrossRef]
- Szcześniak, M.; Grimling, B.; Meler, J.; Karolewicz, B. Application of chitosan in the formulation of dermatological hydrogels prepared on the basis of macromolecular compounds. Prog. Chem. Appl. Chitin its Deriv. 2018, 23, 179–184. [Google Scholar] [CrossRef]
- Pekař, M. Hydrogels with micellar hydrophobic (nano) domains. Front. Mater. 2015, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Sievers, J.; Sperlich, K.; Stahnke, T.; Kreiner, C.; Eickner, T.; Martin, H.; Guthoff, R.F.; Schünemann, M.; Bohn, S.; Stachs, O. Determination of hydrogel swelling factors by two established and a novel non-contact continuous method. J. Appl. Polym. Sci. 2021, 138, 50326. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and effect of structure on swelling properties of hydrogels based on high methylated pectin and acrylic polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Pourjavadi, A.; Barzegar, S. Synthesis and Evaluation of pH and Thermosensitive Pectin-Based Superabsorbent Hydrogel for Oral Drug Delivery Systems. Starch-Stärke 2009, 61, 161–172. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, B.; Rao, J. Pea protein isolate–high methoxyl pectin soluble complexes for improving pea protein functionality: Effect of pH, biopolymer ratio and concentrations. Food Hydrocoll. 2018, 80, 245–253. [Google Scholar] [CrossRef]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Ayub, G.; Naseem, S.; Ansari, M.T. Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J. Mater. Sci. Mater. Med. 2010, 21, 2805–2816. [Google Scholar] [CrossRef]
- Okudan, A.; Altay, A. Investigation of the effects of different hydrophilic and hydrophobic comonomers on the volume phase transition temperatures and thermal properties of N-isopropylacrylamide-based hydrogels. Int. J. Polym. Sci. 2019, 2019, 7324181. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Quintanilla de Stéfano, J.C.; Abundis-Correa, V.; Herrera-Flores, S.D.; Alvarez, A.J. PH-sensitive starch-based hydrogels: Synthesis and effect of molecular components on drug release behavior. Polymers 2020, 12, 1974. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Sánchez-López, E.; Marqués, A.M.; Badia, J.; Baldomà, L.; Espina, M.; Calpena, A.C.; García, M.L. Surface-Modified Multifunctional Thymol-Loaded Biodegradable Nanoparticles for Topical Acne Treatment. Pharmaceutics 2021, 13, 1501. [Google Scholar] [CrossRef]
- Limcharoen, B.; Pisetpackdeekul, P.; Toprangkobsin, P.; Thunyakitpisal, P.; Wanichwecharungruang, S.; Banlunara, W. Topical Proretinal Nanoparticles: Biological Activities, Epidermal Proliferation and Differentiation, Follicular Penetration, and Skin Tolerability. ACS Biomater. Sci. Eng. 2020, 6, 1510–1521. [Google Scholar] [CrossRef]
- Bostanudin, M.F.; Lalatsa, A.; Górecki, D.C.; Barbu, E. Engineering butylglyceryl-modified polysaccharides towards nanomedicines for brain drug delivery. Carbohydr. Polym. 2020, 236, 116060. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Vavrova, K.; Zbytovska, J.; Hrabalek, A. Amphiphilic Transdermal Permeation Enhancers: Structure-Activity Relationships. Curr. Med. Chem. 2005, 12, 2273–2291. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostanudin, M.F. Amphiphilic Alkylated Pectin Hydrogels for Enhanced Topical Delivery of Fusidic Acid: Formulation and In Vitro Investigation. Sci. Pharm. 2022, 90, 13. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90010013
Bostanudin MF. Amphiphilic Alkylated Pectin Hydrogels for Enhanced Topical Delivery of Fusidic Acid: Formulation and In Vitro Investigation. Scientia Pharmaceutica. 2022; 90(1):13. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90010013
Chicago/Turabian StyleBostanudin, Mohammad F. 2022. "Amphiphilic Alkylated Pectin Hydrogels for Enhanced Topical Delivery of Fusidic Acid: Formulation and In Vitro Investigation" Scientia Pharmaceutica 90, no. 1: 13. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90010013