Quality by Design: A Suitable Methodology in Industrial Pharmacy for Costa Rican Universities
Abstract
:1. Introduction
2. University’s Research Model in Costa Rica
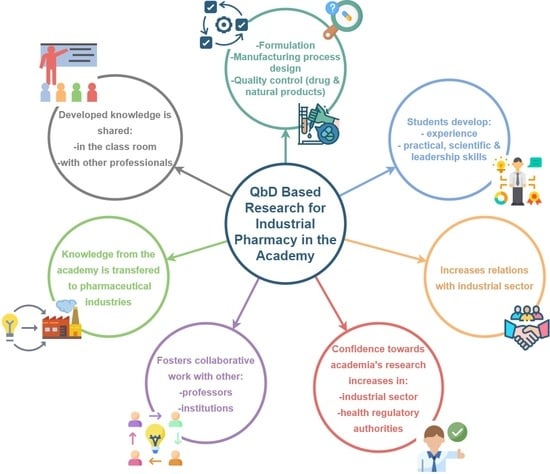
3. Quality by Design Approach for Industrial Pharmacy in Costa Rican Academy
3.1. Model’s Basic Characteristics
3.2. Implementation in Academic Research
3.2.1. Formulation Development
3.2.2. Manufacturing Process Design
3.2.3. Quality Control
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aithal, P.S.; Kumar, P.M. Opportunities and Challenges for Private Universities in India; Social Science Research Network: Rochester, NY, USA, 2016. [Google Scholar]
- Berg, M.; Seeber, B.K. The Slow Professor: Challenging the Culture of Speed in the Academy; University of Toronto Press: Toronto, TO, Canada, 2016; ISBN 978-1-4426-6310-7. [Google Scholar]
- Morales-López, Y. Costa Rica: The Preparation of Mathematics Teachers. In Mathematics Teacher Preparation in Central America and the Caribbean: The Cases of Colombia, Costa Rica, the Dominican Republic and Venezuela; Ruiz, A., Ed.; SpringerBriefs in Education; Springer International Publishing: Cham, Switzerland, 2017; pp. 39–56. ISBN 978-3-319-44177-1. [Google Scholar]
- Morales, R.A. Location of the University Regionalization in Costa Rica. Congreso Universidad. 2016, 5. Available online: http://www.congresouniversidad.cu/revista/index.php/congresouniversidad/index (accessed on 6 December 2021).
- Law, M.; Bader, L.; Uzman, N.; Williams, A.; Bates, I. The FIP Nanjing Statements: Shaping Global Pharmacy and Pharmaceutical Sciences Education. Res. Soc. Adm. Pharm. 2019, 15, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.; DeSilva, B.; Dressman, J.; Ito, M.; Kumamoto, T.; Mager, D.; Mahler, H.-C.; Maitland-van der Zee, A.H.; Pauletti, G.M.; Sasaki, H.; et al. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J. Pharm. Sci. 2016, 105, 2489–2497. [Google Scholar] [CrossRef] [Green Version]
- García Aponte, O.F.; Vallejo Díaz, B.M.; Mora Huertas, C.E. La calidad desde el diseño: Principios y oportunidades para la industria farmacéutica. Estud. Gerenc. 2015, 31, 68–78. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Tahir, M.A.; Charoo, N.A.; Ansari, S.H.; Ali, J. Pharmaceutical Product Development: A Quality by Design Approach. Int. J. Pharm. Investig. 2016, 6, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Juran, J. Juran on Quality by Design; The Free Press: New York, NY, USA, 1992. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding Pharmaceutical Quality by Design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by Design Approach: Regulatory Need. Arab. J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef] [Green Version]
- Grangeia, H.B.; Silva, C.; Simões, S.P.; Reis, M.S. Quality by Design in Pharmaceutical Manufacturing: A Systematic Review of Current Status, Challenges and Future Perspectives. Eur. J. Pharm. Biopharm. 2020, 147, 19–37. [Google Scholar] [CrossRef]
- Bentley, P.J.; Gulbrandsen, M.; Kyvik, S. The Relationship between Basic and Applied Research in Universities. High Educ. 2015, 70, 689–709. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Javaid, M.; Vaishya, R.; Deshmukh, S.G. Areas of Academic Research with the Impact of COVID-19. Am. J. Emerg. Med. 2020, 38, 1524–1526. [Google Scholar] [CrossRef]
- Naidu, P.; Derani, N.E.S. A Comparative Study on Quality of Education Received by Students of Private Universities versus Public Universities. Procedia Econ. Financ. 2016, 35, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Chang, M. Principles of Scientific Methods; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016; ISBN 978-0-429-17190-1. [Google Scholar]
- Pagliaro, M. Enhancing the Use of E-Mail in Scientific Research and in the Academy. Heliyon 2020, 6, e03087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkins, S.; Neri, S.; Lean, J. The Role of Theory in the Business/Management PhD: How Students May Use Theory to Make an Original Contribution to Knowledge. Int. J. Manag. Educ. 2019, 17, 100316. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Brand, J.C.; Rossi, M.J. Medical Device and Pharmaceutical Industry Employees as Medical Research Publication Authors. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 2745–2747. [Google Scholar] [CrossRef] [Green Version]
- Beall, J. Pharmacy Research and Predatory Journals: Authors Beware. Am. J. Health-Syst. Pharm. 2016, 73, 1548–1550. [Google Scholar] [CrossRef]
- Egri, N.; Ortiz de Landazuri, I.; San Bartolomé, C.; Ortega, J.R.; Español-Rego, M.; Juan, M. CART Manufacturing Process and Reasons for Academy-Pharma Collaboration. Immunol. Lett. 2020, 217, 39–48. [Google Scholar] [CrossRef]
- Salau, O.; Osibanjo, A.; Adeniji, A.; Oludayo, O.; Falola, H.; Igbinoba, E.; Ogueyungbo, O. Data Regarding Talent Management Practices and Innovation Performance of Academic Staff in a Technology-Driven Private University. Data Brief 2018, 19, 1040–1045. [Google Scholar] [CrossRef]
- Stewart, D.; Klein, S. The Use of Theory in Research. Int. J. Clin. Pharm. 2016, 38, 615–619. [Google Scholar] [CrossRef] [Green Version]
- Ware, M.; Mabe, M. The STM Report: An Overview of Scientific and Scholarly Journal Publishing. In Copyright, Fair Use, ScholarlyCommunication, etc.; University of Nebraska: Lincoln, NE, USA, 2015. [Google Scholar]
- Pineda, M.A. Cómo comunicar la investigación desde la academia. Rev. Cienc. Tecnol. 2019, 24, 13–15. [Google Scholar] [CrossRef] [Green Version]
- García-Peñalvo, F.J. Publishing in Open Access. J. Inf. Technol. Res. 2017, 10, vi–viii. [Google Scholar]
- Tapasco, O.A.; Giraldo, J.A. Estudio Comparativo Sobre Percepción y Uso de Las TIC Entre Profesores de Universidades Públicas y Privadas. Form. Univ. 2017, 10, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Saez, R.; Gustafsson, R.; Van den Brande, L. The Dawn of an Open Exploration Era: Emergent Principles and Practices of Open Science and Innovation of University Research Teams in a Digital World. Technol. Forecast. Soc. Change 2020, 156, 120037. [Google Scholar] [CrossRef]
- Ahluwalia, A.K.; Preet, K. The Influence of Organizational Commitment on Work Motivation: A Comparative Study of State and Private University Teachers. IUP J. Organ. Behav. 2017, 16, 55–69. [Google Scholar]
- Asaduzzaman, M.; Hossain, M.; Rahman, M. Service Quality and Student Satisfaction: A Case Study on Private Universities in Bangladesh. Int. J. Econ. Financ. Manag. Sci. 2014, 1, 128–135. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, G.; Guan, Z. Estimating the Multi-Period Efficiency of High-Tech Research Institutes of the Chinese Academy of Sciences: A Dynamic Slacks-Based Measure. Socio-Econ. Plan. Sci. 2020, 71, 100855. [Google Scholar] [CrossRef]
- Gayá, P.; Brydon-Miller, M. Carpe the Academy: Dismantling Higher Education and Prefiguring Critical Utopias through Action Research. Futures 2017, 94, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Gómez Vargas, M.; Galeano Higuita, C.; Jaramillo Muñoz, D.A. El estado del arte: Una metodología de investigación. Rev. Colomb. Cienc. Soc. 2015, 6, 423–442. [Google Scholar] [CrossRef]
- Stein, K. Propelling the Profession with Outcomes and Evidence: Building a Robust Research Agenda at the Academy. J. Acad. Nutr. Diet. 2016, 116, 1014–1030. [Google Scholar] [CrossRef]
- Koshkina, I.; Sharamko, M. Economic Security and Internal Control of the Academic Research Projects. Procedia Soc. Behav. Sci. 2015, 214, 858–865. [Google Scholar] [CrossRef] [Green Version]
- International Conference on Harmonisation(ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Q8(R2) Pharmaceutical Development; International Conference on Harmonisation: Geneva, Switzerland, 2009. [Google Scholar]
- Food and Drug Administration Guidance for Industry; U.S. Department of Health and Human Services. Guidance for Industry: Quality Systems Approach to Pharmaceutical CGMP Regulations. 2006. Available online: https://www.fda.gov/media/71023/download (accessed on 5 January 2022).
- Pallagi, E.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. Adaptation of the Quality by Design Concept in Early Pharmaceutical Development of an Intranasal Nanosized Formulation. Int. J. Pharm. 2015, 491, 384–392. [Google Scholar] [CrossRef]
- Rahman, M.; Beg, S.; Panda, S.S. Pharmaceutical QbD: Omnipresence in the Product Development Lifecycle. Eur. Pharm. Rev. 2017, 22, 58–64. [Google Scholar]
- Fornaguera, C.; García-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulhammer, E.; Fink, M.; Llusa, M.; Lawrence, S.M.; Biserni, S.; Calzolari, V.; Khinast, J.G. Low-Dose Capsule Filling of Inhalation Products: Critical Material Attributes and Process Parameters. Int. J. Pharm. 2014, 473, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Manzon, D.; Claeys-Bruno, M.; Declomesnil, S.; Carité, C.; Sergent, M. Quality by Design: Comparison of Design Space Construction Methods in the Case of Design of Experiments. Chemom. Intell. Lab. Syst. 2020, 200, 104002. [Google Scholar] [CrossRef]
- Deepika, P.; Manisha, S.; Parijat, P.; Swagat, T.; Harish, D. Implementation of Quality by Design: A Review. Appl. Clin. Res. Clin. Trials Regul. Aff. Discontin. 2019, 6, 99–111. [Google Scholar]
- Castillo, L.; Zúñiga, V.; Carazo, G.; Calvo, B.; Baltodano, E. Development of Immediate Release Rupatadine Fumarate 10 Mg Tablets: A Quality by Design (QbD) Approach. Drug Dev. Ind. Pharm. 2019, 45, 1674–1681. [Google Scholar] [CrossRef]
- Mishra, V.; Thakur, S.; Patil, A.; Shukla, A. Quality by Design (QbD) Approaches in Current Pharmaceutical Set-Up. Expert. Opin. Drug Deliv. 2018, 15, 737–758. [Google Scholar] [CrossRef]
- Singh, G. Chapter 5—Target Product Profile and Clinical Development Plan. In Pharmaceutical Medicine and Translational Clinical Research; Vohora, D., Singh, G., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 65–80. ISBN 978-0-12-802103-3. [Google Scholar]
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Q9 Quality Risk Management; International Conference on Harmonisation: Geneva, Switzerland, 2005. [Google Scholar]
- Chen, J.; Nichols, B.L.B.; Norris, A.M.; Frazier, C.E.; Edgar, K.J. All-Polysaccharide, Self-Healing Injectable Hydrogels Based on Chitosan and Oxidized Hydroxypropyl Polysaccharides. Biomacromolecules 2020, 21, 4261–4272. [Google Scholar] [CrossRef]
- Stocker, E.; Becker, K.; Hate, S.; Hohl, R.; Schiemenz, W.; Sacher, S.; Zimmer, A.; Salar-Behzadi, S. Application of ICH Q9 Quality Risk Management Tools for Advanced Development of Hot Melt Coated Multiparticulate Systems. J. Pharm. Sci. 2017, 106, 278–290. [Google Scholar] [CrossRef] [Green Version]
- Coccia, M. The Fishbone Diagram to Identify, Systematize and Analyze the Sources of General Purpose Technologies; Social Science Research Network: Rochester, NY, USA, 2018. [Google Scholar]
- Paciarotti, C.; Mazzuto, G.; D’Ettorre, D. A Revised FMEA Application to the Quality Control Management. Int. J. Qual. Reliab. Manag. 2014, 31, 788–810. [Google Scholar] [CrossRef]
- He, Z.; Zhou, P.; Zhang, M.; Goh, T.N. A Review of Analysis of Dynamic Response in Design of Experiments. Qual. Reliab. Eng. Int. 2015, 31, 535–542. [Google Scholar] [CrossRef]
- Politis, S.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of Experiments (DoE) in Pharmaceutical Development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L. Design of Experiments for the Establishment of the Dissolution Test Conditions of Rupatadine Fumarate 10 mg Tablets. J. Drug Deliv. Ther. 2019, 9, 331–336. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of Experiments for Microencapsulation Applications: A Review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline Cellulose, a Direct Compression Binder in a Quality by Design Environment—A Review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Kopp, J.; Zauner, F.B.; Pell, A.; Hausjell, J.; Humer, D.; Ebner, J.; Herwig, C.; Spadiut, O.; Slouka, C.; Pell, R. Development of a Generic Reversed-Phase Liquid Chromatography Method for Protein Quantification Using Analytical Quality-by-Design Principles. J. Pharm. Biomed. Anal. 2020, 188, 113412. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, T.I.; Sivadier, G.; Gaudin, K. Development of a Green HPLC Method for the Analysis of Artesunate and Amodiaquine Impurities Using Quality by Design. J. Pharm. Biomed. Anal. 2020, 190, 113507. [Google Scholar] [CrossRef]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical Quality by Design: Development and Control Strategy for a LC Method to Evaluate the Cannabinoids Content in Cannabis Olive Oil Extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef]
- Yekpe, K.; Abatzoglou, N.; Bataille, B.; Gosselin, R.; Sharkawi, T.; Simard, J.-S.; Cournoyer, A. Developing a Quality by Design Approach to Model Tablet Dissolution Testing: An Industrial Case Study. Pharm. Dev. Technol. 2018, 23, 646–654. [Google Scholar] [CrossRef]
- Nadella, N.P.; Ratnakaram, V.N.; Srinivasu, N. Quality-by-Design-Based Development and Validation of a Stability-Indicating UPLC Method for Quantification of Teriflunomide in the Presence of Degradation Products and Its Application to in-Vitro Dissolution. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 517–527. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Siddiqui, S.; Rao, S.; Mohanty, P.; Ara, T.J.; Beg, S. QbD-Driven Development and Validation of a Bioanalytical LC-MS Method for Quantification of Fluoxetine in Human Plasma. J Chromatogr. Sci. 2016, 54, 736–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.F.; Silva, G.; Pinto, A.C.; Fonseca, M.; Silva, N.E.; Pinto, R.M.A.; Simões, S. Artificial Neural Networks Applied to Quality-by-Design: From Formulation Development to Clinical Outcome. Eur. J. Pharm. Biopharm. 2020, 152, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Bandari, S.; Repka, M.A. Quality-by-Design in Hot Melt Extrusion Based Amorphous Solid Dispersions: An Industrial Perspective on Product Development. Eur. J. Pharm. Sci. 2021, 158, 105655. [Google Scholar] [CrossRef]
- Gurumukhi, V.C.; Bari, S.B. Fabrication of Efavirenz Loaded Nano-Formulation Using Quality by Design (QbD) Based Approach: Exploring Characterizations and in Vivo Safety. J. Drug Deliv. Sci. Technol. 2020, 56, 101545. [Google Scholar] [CrossRef]
- Patwardhan, D.; Amrutkar, S.; Kotwal, T.; Wagh, P. Application of Quality by Design to Different Aspects of Pharmaceutical Technologies. Int. J. Pharm. Sci. Res. 2017, 5, 3649–3662. [Google Scholar]
- Peraman, R.; Bhadraya, K.; Padmanabha Reddy, Y. Analytical Quality by Design: A Tool for Regulatory Flexibility and Robust Analytics. Int. J. Anal. Chem. 2015, 2015, e868727. [Google Scholar] [CrossRef]
- Bai, G.; Chen, Z.; Raines, K.; Chen, H.; Dave, K.; Lin, H.-P.; Zolnik, B.S. Assessment of Applications of Design of Experiments in Pharmaceutical Development for Oral Solid Dosage Forms. J. Pharm. Innov. 2020, 15, 547–555. [Google Scholar] [CrossRef]
- Orozco, J.; Ruiz, K. Contributions of Universities and Public Research Centers to Innovation Processes in the Industry: The Costa Rican Case; Georgia Institute of Technology: Atlanta, GA, USA, 2009. [Google Scholar]
- Jagan, B.G.V.S.; Mahapatra, A.K.; Murthy, N.P.; Patra, R.K. Quality by Design (QbD): Principles, Underlying Concepts and Regulatory Prospects. Thai J. Pharm. Sci. (TJPS) 2021, 45, 54–69. [Google Scholar]
- Rahman, Z.; Xu, X.; Katragadda, U.; Krishnaiah, Y.S.R.; Yu, L.; Khan, M.A. Quality by Design Approach for Understanding the Critical Quality Attributes of Cyclosporine Ophthalmic Emulsion. Mol. Pharm. 2014, 11, 787–799. [Google Scholar] [CrossRef]
- Oh, G.-H.; Park, J.-H.; Shin, H.-W.; Kim, J.-E.; Park, Y.-J. Quality-by-Design Approach for the Development of Telmisartan Potassium Tablets. Drug Dev. Ind. Pharm. 2018, 44, 837–848. [Google Scholar] [CrossRef]
- SimonoskaCrcarevska, M.; Dimitrovska, A.; Sibinovska, N.; Mladenovska, K.; SlavevskaRaicki, R.; GlavasDodov, M. Implementation of Quality by Design Principles in the Development of Microsponges as Drug Delivery Carriers: Identification and Optimization of Critical Factors Using Multivariate Statistical Analyses and Design of Experiments Studies. Int. J. Pharm. 2015, 489, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, L.; Wan, F.; Bera, H.; Cun, D.; Rantanen, J.; Yang, M. Quality by Design Thinking in the Development of Long-Acting Injectable PLGA/PLA-Based Microspheres for Peptide and Protein Drug Delivery. Int. J. Pharm. 2020, 585, 119441. [Google Scholar] [CrossRef] [PubMed]
- Alt, N.; Zhang, T.Y.; Motchnik, P.; Taticek, R.; Quarmby, V.; Schlothauer, T.; Beck, H.; Emrich, T.; Harris, R.J. Determination of Critical Quality Attributes for Monoclonal Antibodies Using Quality by Design Principles. Biologicals 2016, 44, 291–305. [Google Scholar] [CrossRef]
- Lee, A.R.; Kwon, S.Y.; Choi, D.H.; Park, E.S. Quality by Design (QbD) Approach to Optimize the Formulation of a Bilayer Combination Tablet (Telmiduo®) Manufactured via High Shear Wet Granulation. Int. J. Pharm. 2017, 534, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Wang, R.; Liu, G.; Liu, X.; Liu, C.; Deng, Y.; Bai, Z. Quality by Design-Driven Process Development of Severe Fever With Thrombocytopenia Syndrome Vaccine. J. Pharm. Sci. 2019, 108, 3785–3791. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Nanda, S. Quality by Design Driven Development of Resveratrol Loaded Ethosomal Hydrogel for Improved Dermatological Benefits via Enhanced Skin Permeation and Retention. Int. J. Pharm. 2019, 567, 118448. [Google Scholar] [CrossRef] [PubMed]
- Alalaiwe, A.; Fayed, M.H.; Alshahrani, S.M.; Alsulays, B.B.; Alshetaili, A.S.; Tawfeek, H.M.; Khafagy, E.-S. Application of Design of Experiment Approach for Investigating the Effect of Partially Pre-Gelatinized Starch on Critical Quality Attributes of Rapid Orally Disintegrating Tablets. J. Drug Deliv. Sci. Technol. 2019, 49, 227–234. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wang, R.; An, F.; Nie, J.; Zhang, Y.; Ahamada, H.; Liu, X.; Liu, C.; Deng, Y.; et al. Quality by Design-Driven Process Development of Cell Culture in Bioreactor for the Production of Foot-And-Mouth Veterinary Vaccine. J. Pharm. Sci. 2019, 108, 2288–2295. [Google Scholar] [CrossRef]
- Ali, R.; Mehta, P.; KyriakiMonou, P.; Arshad, M.S.; Panteris, E.; Rasekh, M.; Singh, N.; Qutachi, O.; Wilson, P.; Tzetzis, D.; et al. Electrospinning/Electrospraying Coatings for Metal Microneedles: A Design of Experiments (DOE) and Quality by Design (QbD) Approach. Eur. J. Pharm. Biopharm. 2020, 156, 20–39. [Google Scholar] [CrossRef]
- Ahsan Hafiz, M.; Abbas, N.; Bukhari, N.I. Quality by Design Approach for Formulation Development and Evaluation of Carboplatin Loaded EthylcelluloseNanosponges. Int. J. Polym. Mater. Polym. Biomater. 2021, 1–13. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.A.; Zidan, A.S.; Rahman, Z. Quality by Design Approach for Formulation Development: A Case Study of Dispersible Tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Jha, A. Quality Risk Management during Pharmaceutical ‘Good Distribution Practices’—A Plausible Solution. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 18–25. [Google Scholar] [CrossRef]
- Westgard, J.O.; Westgard, S.A. Six Sigma Quality Management System and Design of Risk-Based Statistical Quality Control. Clin. Lab. Med. 2017, 37, 85–96. [Google Scholar] [CrossRef]
- Hanley, G. Desarrollo de una Suspensión Oral con Efecto Anti-Ulceroso y Protector Gástrico Mediante el Modelo de Calidad por Diseño en Laboratorios Raven S.A. Bachelor’s Thesis, Facultad de Farmacia, Universidad de Costa Rica, San Pedro, Costa Rica, 2021. [Google Scholar]
- Fukuda, I.M.; Pinto, C.F.F.; dos Santos Moreira, C.; Saviano, A.M.; Lourenço, F.R. DesignofExperiments (DoE) AppliedtoPharmaceutical and AnalyticalQualitybyDesign (QbD). Braz. J. Pharm. Sci. 2018, 54. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmed, S.A.; Khatoon, A.; Afzal, M.; Ansari, M.T.; Khatoon, S.; Tabish, M.; Al-Marshad, F.M.; Nayak, A.K. Chapter 4—Pharmaceutical Product Development: A Quality by Design (QbD) Approach. In Advances and Challenges in Pharmaceutical Technology; Nayak, A.K., Pal, K., Banerjee, I., Maji, S., Nanda, U., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 131–146. ISBN 978-0-12-820043-8. [Google Scholar]
- Dhoot, A.S.; Fernandes, G.J.; Naha, A.; Rathnanand, M.; Kumar, L. Design of Experiments in Pharmaceutical Development. Pharm. Chem. J. 2019, 53, 730–735. [Google Scholar] [CrossRef]
- Ramírez, J. Preformulación de un Medicamento de Liberación Prolongada Mediante el Enfoque de Calidad por Diseño en Laboratorios Medigray Durante el Segundo y Tercer Cuatrimestre 2021. Bachelor’s Thesis, Facultad de Farmacia, Universidad Internacional de Las Américas, San José, Costa Rica, 2021. [Google Scholar]
- Castillo-Henríquez, L.; Sanabria-Espinoza, P.; Murillo-Castillo, B.; Montes de Oca-Vásquez, G.; Batista-Menezes, D.; Calvo-Guzmán, B.; Ramírez-Arguedas, N.; Vega-Baudrit, J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics 2021, 13, 2100. [Google Scholar] [CrossRef]
- Rathore, A.S.; Winkle, H. Quality by Design for Biopharmaceuticals. Nat. Biotechnol. 2009, 27, 26–34. [Google Scholar] [CrossRef]
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Q11 Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities); International Conference on Harmonisation: Geneva, Switzerland, 2012. [Google Scholar]
- International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Q13 Continuous Manufacturing of Drug Substances and Drug Products; International Conference on Harmonisation: Geneva, Switzerland, 2018. [Google Scholar]
- Department of Health and Human Service—FDA. Guidance for Industry PAT—A Framework for Innovative Pharmaceutical Development, Manufacuring, and Quality Assurance; Food and Drug Administration: Silver Spring, MD, USA, 2004. [Google Scholar]
- Reklaitis, G.V.; Khinast, J.; Muzzio, F. Pharmaceutical Engineering Science—New Approaches to Pharmaceutical Development and Manufacturing. Chem. Eng. Sci. 2010, 65, iv–vii. [Google Scholar] [CrossRef]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A Perspective on Quality-by-Control (QbC) in Pharmaceutical Continuous Manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]
- Yu, L.X.; Kopcha, M. The Future of Pharmaceutical Quality and the Path to Get There. Int. J. Pharm. 2017, 528, 354–359. [Google Scholar] [CrossRef]
- Cantillo, G. Diseño del Proceso de Recubrimiento Pelicular de Tabletas a Escala Piloto Mediante la Aplicación del Modelo de Calidad por Diseño en el Departamento de Investigación y Desarrollo en Laboratorios Infarma LTDA. Bachelor’s Thesis, Facultad de Farmacia, Universidad de Costa Rica, San Pedro, Costa Rica, 2018. [Google Scholar]
- Raman, N.V.V.S.S.; Mallu, U.R.; Bapatu, H.R. Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing. J. Chem. 2015, 2015, e435129. [Google Scholar] [CrossRef]
- Vogt, F.G.; Kord, A.S. Development of Quality-By-Design Analytical Methods. J. Pharm. Sci. 2011, 100, 797–812. [Google Scholar] [CrossRef]
- Murillo, B. Desarrollo y Validación de un Método Bioanalítico por Cromatografía Líquida de Alta Resolución con Detector de Arreglo de Diodos para la Cuantificación Simultánea de Carbamazepina y su Metabolito Activo Carbamazepina 10,11 Epóxido en Plasma Humano. Bachelor’s Thesis, Facultad de Farmacia, Universidad de Costa Rica, San Pedro, Costa Rica, 2021. [Google Scholar]
- Rodino, S.; Butu, M. 3—Herbal Extracts—New Trends in Functional and Medicinal Beverages. In Functional and Medicinal Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 73–108. ISBN 978-0-12-816397-9. [Google Scholar]
- Prajapati, P.; Patel, H.; Shah, S. Quality risk assessment and DoE-based analytical quality by design approach to stability-indicating assay method for acidic degradation kinetic study of apremilast. J. Planar Chromatogr. 2020, 33, 231–244. [Google Scholar] [CrossRef]
- Castillo, L.; Baltodano, E.; Ramírez, N.; Vargas, R.; Hanley, G. Design of Experiments Assessment for the Determination of Moisture Content in Five Herbal Raw Materials Contained in Tea Products. Borneo J. Pharm. 2020, 3, 22–35. [Google Scholar] [CrossRef]






| Application | Purpose | Ref. |
|---|---|---|
| Microcrystalline cellulose for direct compression | Excipient development | [56] |
| Cyclosporine ophthalmic emulsion | Bioequivalence method validation | [71] |
| Validation of a bioanalytical method for quantification of fluoxetine in human plasma | [62] | |
| Telmisartan potassium tablets | [72] | |
| Development of microsponges using double emulsion solvent diffusion technique | Formulation development/optimization | [73] |
| Development of long-acting injectable PLGA/PLA-based microspheres | [74] | |
| Determination of critical quality attributes for monoclonal antibodies | Biotechnological drug analysis | [75] |
| Development of a reversed-phase liquid chromatography method for protein quantification | [57] | |
| Formulation of a bilayer combined tablet manufactured via high-shear wet granulation | Formulation/process optimization | [76] |
| Ultraperformance liquid chromatography method for quantification of teriflunomide | Dissolution and stability testing | [61] |
| Development of Bunyavirus vaccine | Process development for biologics manufacturing | [77] |
| Development of resveratrol-loaded ethosomal hydrogel | Dermal delivery system | [78] |
| Determination of partially pre-gelatinized starch effect on rapid orally disintegrating tablets | Identification of CQA | [79] |
| Development of green HPLC method for artesunate and amodiaquine impurities | Quality control | [58] |
| Liquid chromatography method to evaluate cannabinoid content in cannabis olive oil extracts | Quality control of natural products | [59] |
| Cell culture in bioreactor for the production of foot-and-mouth veterinary vaccine | Biopharmaceutical process development | [80] |
| Development of electrospinning coatings for metal microneedles | Process optimization | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo-Henríquez, L.; Murillo-Castillo, B.; Chaves-Siles, L.; Mora-Román, J.J.; Ramírez-Arguedas, N.; Hernández-Mora, É.; Vega-Baudrit, J. Quality by Design: A Suitable Methodology in Industrial Pharmacy for Costa Rican Universities. Sci. Pharm. 2022, 90, 34. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90020034
Castillo-Henríquez L, Murillo-Castillo B, Chaves-Siles L, Mora-Román JJ, Ramírez-Arguedas N, Hernández-Mora É, Vega-Baudrit J. Quality by Design: A Suitable Methodology in Industrial Pharmacy for Costa Rican Universities. Scientia Pharmaceutica. 2022; 90(2):34. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90020034
Chicago/Turabian StyleCastillo-Henríquez, Luis, Brayan Murillo-Castillo, Lexi Chaves-Siles, Juan José Mora-Román, Nils Ramírez-Arguedas, Édgar Hernández-Mora, and José Vega-Baudrit. 2022. "Quality by Design: A Suitable Methodology in Industrial Pharmacy for Costa Rican Universities" Scientia Pharmaceutica 90, no. 2: 34. https://0-doi-org.brum.beds.ac.uk/10.3390/scipharm90020034