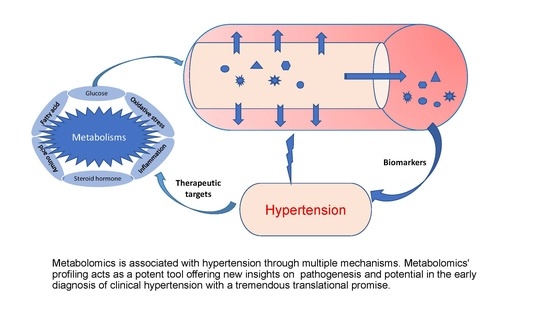
Metabolic Profiling and Metabolites Fingerprints in Human Hypertension: Discovery and Potential
Abstract
:1. Introduction
2. Metabolomics and Metabolite Profiling/Fingerprinting
3. Metabolomic Fingerprinting in Hypertension and the Associated Mechanisms
3.1. Glucose Metabolism
3.2. Amino Acids Metabolism
3.3. Fatty Acids Metabolism
3.4. Oxidative Stress
3.5. Inflammation
3.6. Steroid Hormones Biosynthesis
4. Limitations, Challenges, and Future Perspective of Metabolomic Fingerprinting in Hypertension
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsiropoulou, S.; McBride, M.; Padmanabhan, S. Hypertension: Methods and Protocols, Methods in Molecular Biology; Touyz, R.M., Schiffrin, E.L., Eds.; Springer: New York, NY, USA, 2017; Volume 1527, pp. 60–68. [Google Scholar]
- Heather, L.C.; Wang, X.; West, J.A.; Griffin, J.L. A practical guide to metabolomic profiling as a discovery tool for human heart disease. J. Mol. Cell. Cardiol. 2013, 55, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.L.; Atherton, H.J.; Shockcor, J.P.; Atzori, L. Metabolomics as a tool for cardiac research. Nat. Rev. Cardiol. 2011, 8, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Iliou, A.; Mikros, E.; Elliott, P. An Overview of Metabolic Phenotyping in Blood Pressure Research. Curr. Hypertens. Rep. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzoulaki, I.; Ebbels, T.M.D.; Valdes, A.; Elliott, P.; Ioannidis, J.P.A. Design and Analysis of Metabolomics Studies in Epidemiologic Research: A Primer on -Omic Technologies. Am. J. Epidemiol. 2014, 180, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Yu, Z.; Deng, S.; Chen, X.; Chen, L.; Guo, Z.; Zheng, H.; Chen, L.; Cai, D.; Wen, B.; et al. A Targeted Metabolomics MRM-MS Study on Identifying Potential Hypertension Biomarkers in Human Plasma and Evaluating Acupuncture Effects. Sci. Rep. 2016, 6, 25871. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.D.; Cruthirds, C.L.; Lockwood, C.M.; Pappan, K.; Childs, T.E.; Company, J.M.; Brown, J.D.; Toedebusch, R.G.; Booth, F.W. Comparing serum responses to acute feedings of an extensively hydrolyzed whey protein concentrate versus a native whey protein concentrate in rats: A metabolomics approach. Appl. Physiol. Nutr. Metab. 2014, 39, 158–167. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Elliott, P.; Kontis, V.; Ezzati, M. Worldwide Exposures to Cardiovascular Risk Factors and Associated Health Effects: Current Knowledge and Data Gaps. Circulation 2016, 133, 2314–2333. [Google Scholar] [CrossRef] [Green Version]
- Mels, C.M.; Delles, C.; Louw, R.; Schutte, A. Central systolic pressure and a nonessential amino acid metabolomics profile. J. Hypertens. 2019, 37, 1157–1166. [Google Scholar] [CrossRef]
- Ameta, K.; Gupta, A.; Kumar, S.; Sethi, R.; Kumar, D.; Mahdi, A.A. Essential hypertension: A filtered serum based metabolomics study. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kearney, P.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Zhou, B.; Bentham, J.; Di Cesare, M.; Bixby, H.; Danaei, G.; Cowan, M.J.; Paciorek, C.J.; Singh, G.; Hajifathalian, K.; Bennett, J.E.; et al. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Sherlock, P.; Beard, J.; Minicuci, N.; Ebrahim, S.; Chatterji, S. Hypertension among older adults in low- and middle-income countries: Prevalence, awareness and control. Int. J. Epidemiol. 2014, 43, 116–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menni, C.; Graham, D.; Kastenmüller, G.; Alharbi, N.H.; AlSanosi, S.M.; McBride, M.; Mangino, M.; Titcombe, P.; Shin, S.-Y.; Psatha, M.; et al. Metabolomic Identification of a Novel Pathway of Blood Pressure Regulation Involving Hexadecanedioate. Hypertension 2015, 66, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brindle, J.T.; Nicholson, J.; Schofield, P.M.; Grainger, D.J.; Holmes, E. Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension. Analyst 2002, 128, 32–36. [Google Scholar] [CrossRef]
- García-Puig, J.; Ruilope, L.M.; Luque, M.; Fernández, J.; Ortega, R.; Dal-Ré, R. Glucose Metabolism in Patients with Essential Hypertension. Am. J. Med. 2006, 119, 318–326. [Google Scholar] [CrossRef]
- Metabolite Profiling and Cardiovascular Event Risk: A Prospective Study of 3 Population-Based Cohorts—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25573147/?from_single_result=Metabolite+profiling+and+cardiovascular+event+risk%3A+a+prospective+study+of+3+population-based+cohorts (accessed on 3 June 2020).
- Gu, D.; Reynolds, K.; Wu, X.; Chen, J.; Duan, X.; Muntner, P.; Huang, G.; Reynolds, R.F.; Su, S.; Whelton, P.K.; et al. Prevalence, Awareness, Treatment, and Control of Hypertension in China. Hypertension 2002, 40, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Ong, K.L.; Cheung, B.M.; Man, Y.B.; Lau, C.P.; Lam, K.S. Prevalence, Awareness, Treatment, and Control of Hypertension Among United States Adults 1999–2004. Hypertension 2007, 49, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Hiltunen, T.P.; Rimpelä, J.; Mohney, R.P.; Stirdivant, S.M.; Kontula, K.K. Effects of four different antihypertensive drugs on plasma metabolomic profiles in patients with essential hypertension. PLoS ONE 2017, 12, e0187729. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Holmes, E.; Kinross, J.M.; Darzi, A.W.; Takats, Z.; Lindon, J.C. Metabolic phenotyping in clinical and surgical environments. Nature 2012, 491, 384–392. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Tan, K.; Sun, X.; Ye, J.; Zhang, Y. Circulating metabolomics profiling reveals novel pathways associated with cognitive decline in patients with hypertension. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420947973. [Google Scholar] [CrossRef]
- Nikolic, S.B.; Sharman, J.; Adams, M.; Edwards, L.M. Metabolomics in hypertension. J. Hypertens. 2014, 32, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Bailey, N.J.C.; Johnson, H.E. Measuring the metabolome: Current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. HMDB: A knowledgebase for the human metabolome. Nucleic Acids Res. 2008, 37, D603–D610. [Google Scholar] [CrossRef] [PubMed]
- Lenz, E.M.; Wilson, I. Analytical Strategies in Metabonomics. J. Proteome Res. 2007, 6, 443–458. [Google Scholar] [CrossRef]
- Theodoridis, G.; Gika, H.G.; Wilson, I.D. LC-MS-based methodology for global metabolite profiling in metabonomics/metabolomics. TrAC Trends Anal. Chem. 2008, 27, 251–260. [Google Scholar] [CrossRef]
- Onuh, J.O.; Aluko, R.E. Metabolomics as a tool to study the mechanism of action of bioactive protein hydrolysates and peptides: A review of current literature. Trends Food Sci. Technol. 2019, 91, 625–633. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Onuh, J.O.; Aliani, M. Metabolomics profiling in hypertension and blood pressure regulation. A review. Clin. Hypertens. 2020, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Peng, B.; Wu, X.; Cao, Y.; Sun, X.; Hong, M.; Na, R.; Liu, B.; Li, Q.; Li, Z.; et al. Metabolomic study for essential hypertension patients based on dried blood spot mass spectrometry approach. IUBMB Life 2018, 70, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polonis, K.; Wawrzyniak, R.; Daghir-Wojtkowiak, E.; Szyndler, A.; Chrostowska, M.; Melander, O.; Hoffmann, M.; Kordalewska, M.; Raczak-Gutknecht, J.; Bartosińska, E.; et al. Metabolomic Signature of Early Vascular Aging (EVA) in Hypertension. Front. Mol. Biosci. 2020, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Salihovic, S.; Fall, T.; Hammar, U.; Ingelsson, E.; Ärnlöv, J.; Lind, L.; Sundström, J. Global Plasma Metabolomics to Identify Potential Biomarkers of Blood Pressure Progression. Arter. Thromb. Vasc. Biol. 2020, 40, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, Y.; Li, Z.; Song, Y.; Cai, X.; Liu, Y.; Zhang, T.; Yang, L.; Li, L.; Gao, S.; et al. Identification of essential hypertension biomarkers in human urine by non-targeted metabolomics based on UPLC-Q-TOF/MS. Clin. Chim. Acta 2018, 486, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Goïta, Y.; De La Barca, J.M.C.; Keïta, A.; Diarra, M.B.; Dembélé, K.C.; Chabrun, F.; Dramé, B.S.I.; Kassogué, Y.; Diakité, M.; Mirebeau-Prunier, D.; et al. Sexual Dimorphism of Metabolomic Profile in Arterial Hypertension. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic characterization of hypertension and dyslipidemia. Metabolomics 2018, 14, 117. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, C.; Su, J.; Pan, C.-W.; Ke, C. Identification of biomarkers for essential hypertension based on metabolomics. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 382–395. [Google Scholar] [CrossRef]
- Boller, S.; Joblin, B.A.; Xu, L.; Item, F.; Trüb, T.; Boschetti, N.; Spinas, G.A.; Niessen, M. From signal transduction to signal interpretation: An alternative model for the molecular function of insulin receptor substrates. Arch. Physiol. Biochem. 2012, 118, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, T.; Qiu, Y.; Cheng, Y.; Cao, Y.; Zhao, A.; Jia, W. An ultrasonication-assisted extraction and derivatization protocol for GC/TOFMS-based metabolite profiling. Anal. Bioanal. Chem. 2011, 400, 1405–1417. [Google Scholar] [CrossRef] [Green Version]
- Maresh, M.; Lawrence, J.M.; Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Deerochanawong, C.; Sacks, D.A.; Lowe, W.L.; Dyer, A.R.; Metzger, B.E. Association of glucose metabolism and blood pressure during pregnancy with subsequent maternal blood pressure. J. Hum. Hypertens. 2021, 1–8. [Google Scholar] [CrossRef]
- Dhar, I.; Lysne, V.; Seifert, R.; Svingen, G.F.; Ueland, P.M.; Nygård, O.K.; Dhar, I. Plasma methionine and risk of acute myocardial infarction: Effect modification by established risk factors. Atherosclerosis 2018, 272, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.; Duggan, G.; Weljie, A.; Hittel, D.S.; Wasserman, D.H.; Vogel, H.J. Metabolomic profiling of dietary-induced insulin resistance in the high fat-fed C57BL/6J mouse. Diabetes Obes. Metab. 2008, 10, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Lee, B.-H.; Cobb, M.; Goldsmith, E.J. Crystal Structure of the Kinase Domain of WNK1, a Kinase that Causes a Hereditary Form of Hypertension. Structure 2004, 12, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Ni, R.; Chu, L.; Xu, D.; Li, Y.; Li, Y.; Zhang, Y.; You, M.; Zhu, Y.; Ouyang, F.; Zhang, J.; et al. Risk factors of cerebral microbleeds in young and middle-aged patients with hypertension. Neurol. Res. 2018, 40, 413–418. [Google Scholar] [CrossRef]
- Ecobici, M.; Stoicescu, C. Arterial Stiffness and Hypertension–Which Comes First? MAEDICA J. Clin. Med. 2017, 12, 184–190. [Google Scholar]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H.; et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Hear. J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schutte, A.; Huisman, H.; Schutte, R.; Van Rooyen, J.M.; Malan, L.; Malan, N.T.; Reimann, M. Arterial Stiffness Profiles: Investigating Various Sections of the Arterial Tree of African and Caucasian People. Clin. Exp. Hypertens. 2011, 33, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.A.; Patel, R.S.; Binongo, J.N.G.; Poole, J.; Al Mheid, I.; Ahmed, Y.; Stoyanova, N.; Vaccarino, V.; Din-Dzietham, R.; Gibbons, G.H.; et al. Racial Differences in Arterial Stiffness and Microcirculatory Function Between Black and White Americans. J. Am. Hear. Assoc. 2013, 2, e002154. [Google Scholar] [CrossRef] [Green Version]
- Schutte, A.; Botha, S.; Fourie, C.M.T.; Gafane-Matemane, L.; Kruger, R.; Lammertyn, L.; Malan, L.; MC Mels, C.; Schutte, R.; Smith, W.; et al. Recent advances in understanding hypertension development in sub-Saharan Africa. J. Hum. Hypertens. 2017, 31, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Nakaki, T.; Hishikawa, K. The arginine paradox. Folia Pharmacol. Jpn. 2002, 119, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Moss, M.B.; Brunini, T.M.C.; De Moura, R.S.; Malagris, L.E.N.; Roberts, N.B.; Ellory, J.C.; Mann, G.E.; Ribeiro, A.C.M. Diminished L-arginine bioavailability in hypertension. Clin. Sci. 2004, 107, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, H.O.; Tarshoby, M.; Monestel, R.; Hook, G.; Cronin, J.; Johnson, A.; Bayazeed, B.; Baron, A.D. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J. Clin. Investig. 1997, 100, 1230–1239. [Google Scholar] [CrossRef]
- Fändriks, L. Roles of the gut in the metabolic syndrome: An overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Kasubuchi, M.; Nakajima, A.; Irie, J.; Itoh, H.; Kimura, I. The role of short-chain fatty acid on blood pressure regulation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.J.; Huang, T.; Cheney, F.W. Effect of vasodilator treatment on the resolution of oleic acid injury in dogs. Am. Rev. Respir. Dis. 1985, 131, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Teres, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escriba, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemecz, M.; Constantin, A.; Dumitrescu, M.; Alexandru, N.; Filippi, A.; Tanko, G.; Georgescu, A. The Distinct Effects of Palmitic and Oleic Acid on Pancreatic Beta Cell Function: The Elucidation of Associated Mechanisms and Effector Molecules. Front. Pharmacol. 2019, 9, 1554. [Google Scholar] [CrossRef] [Green Version]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr. Protoc. Mol. Biol. 2012, 98, 30.2.1–30.2.24. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, J.; Lu, C.; Hsin, M.; Mura, M.; Wu, L.; Chu, L.; Zamel, R.; Machuca, T.; Waddell, T.; et al. Metabolomic Heterogeneity of Pulmonary Arterial Hypertension. PLoS ONE 2014, 9, e88727. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, P.M.; Boutouyrie, P.; Laurent, S. Vascular Aging. Hypertension 2009, 54, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic Stiffness, Blood Pressure Progression, and Incident Hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Dudenbostel, T.; Glasser, S. Effects of Antihypertensive Drugs on Arterial Stiffness. Cardiol. Rev. 2012, 20, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavi, S.; McConnell, J.P.; Rihal, C.S.; Prasad, A.; Mathew, V.; Lerman, L.O.; Lerman, A. Local Production of Lipoprotein-Associated Phospholipase A 2 and Lysophosphatidylcholine in the Coronary Circulation. Circulation 2007, 115, 2715–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Kim, D.H.; Jung, K.J.; Heo, H.-S.; Kim, C.H.; Baik, H.S.; Yu, B.P.; Yokozawa, T.; Chung, H.Y. Lysophosphatidylcholine Enhances Oxidative Stress Via the 5-Lipoxygenase Pathway in Rat Aorta During Aging. Rejuvenation Res. 2009, 12, 15–24. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on autoimmunity and oxidative stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Vargas, F.; Gómez, I.M.R.; Pérez-Abud, R.; Tendero, P.V.; Baca, Y.; Wangensteen, R.; Fuentes, R.W. Cardiovascular and renal manifestations of glutathione depletion induced by buthionine sulfoximine. Am. J. Hypertens. 2012, 25, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Onuh, J.O.; Girgih, A.T.; Nwachukwu, I.; Ievari-Shariati, S.; Raj, P.; Netticadan, T.; Aluko, R.E.; Aliani, M. A metabolomics approach for investigating urinary and plasma changes in spontaneously hypertensive rats (SHR) fed with chicken skin protein hydrolysates diets. J. Funct. Foods 2016, 22, 20–33. [Google Scholar] [CrossRef]
- Cicero, A.F.; Salvi, P.; D’Addato, S.; Rosticci, M.; Borghi, C. Association between serum uric acid, hypertension, vascular stiffness and subclinical atherosclerosis. J. Hypertens. 2014, 32, 57–64. [Google Scholar] [CrossRef]
- Lochner, A.; Genade, S.; Davids, A.; Ytrehus, K.; Moolman, J.A. Short- and long-term effects of melatonin on myocardial post-ischemic recovery. J. Pineal Res. 2006, 40, 56–63. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Paredes, S.D.; Fuentes-Broto, L. Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 2010, 42, 276–285. [Google Scholar] [CrossRef]
- Paapstel, K.; Kals, J.; Eha, J.; Tootsi, K.; Ottas, A.; Piir, A.; Jakobson, M.; Lieberg, J.; Zilmer, M. Inverse relations of serum phosphatidylcholines and lysophosphatidylcholines with vascular damage and heart rate in patients with atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 44–52. [Google Scholar] [CrossRef]
- Caillon, A.; Schiffrin, E.L. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr. Hypertens. Rep. 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Sun, G.Y.; Shelat, P.B.; Jensen, M.B.; He, Y.; Sun, A.Y.; Simonyi, A. Phospholipases A2 and Inflammatory Responses in the Central Nervous System. Neuromolecular Med. 2009, 12, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, T.; Kamata, K. Role of Lysophosphatidylcholine (LPC) in Atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668 (Suppl. S1), S50–S58. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.J.; Li, C.; Mi, X.; Shi, M.; Gu, X.; Bazzano, L.A.; Razavi, A.C.; Nierenberg, J.; Dorans, K.; He, H.; et al. An untargeted metabolomics study of blood pressure: Findings from the Bogalusa Heart Study. J. Hypertens. 2020, 38, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.; Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Otsuki, T.; Kozu, K.; Numano, K.; Suzuki, K.; et al. Protective Roles of Endothelial AMP-Activated Protein Kinase Against Hypoxia-Induced Pulmonary Hypertension in Mice. Circ. Res. 2016, 119, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Graessler, J.; Schwudke, D.; Schwarz, P.E.H.; Herzog, R.; Shevchenko, A.; Bornstein, S.R. Top-Down Lipidomics Reveals Ether Lipid Deficiency in Blood Plasma of Hypertensive Patients. PLoS ONE 2009, 4, e6261. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Lurbe, E.; Laurent, S. The early life origins of vascular ageing and cardiovascular risk: The EVA syndrome. J. Hypertens. 2008, 26, 1049–1057. [Google Scholar] [CrossRef]
- Schmitz, G.; Ruebsaamen, K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 2010, 208, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.M.; Chisolm, G.M. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J. Lipid Res. 2000, 41, 1188–1198. [Google Scholar] [CrossRef]
- Falkner, B.; Daniels, S.R. Summary of the Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Hypertension 2004, 44, 387–388. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, J.E.; Geller, D.S. Glucocorticoid-induced hypertension. Pediatr. Nephrol. 2011, 27, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Panin, L.E.; Mokrushnikov, P.V.; Kunitsyn, V.G.; Zaitsev, B.N. Interaction Mechanism of Anabolic Steroid Hormones with Structural Components of Erythrocyte Membranes. J. Phys. Chem. B 2011, 115, 14969–14979. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Carling, D. AMP-activated protein kinase: The current landscape for drug development. Nat. Rev. Drug Discov. 2019, 18, 527–551. [Google Scholar] [CrossRef]
- Quagliariello, V.; De Laurentiis, M.; Rea, D.; Barbieri, A.; Monti, M.G.; Carbone, A.; Paccone, A.; Altucci, L.; Conte, M.; Canale, M.L.; et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc. Diabetol. 2021, 20, 150. [Google Scholar] [CrossRef]
- James, E.; Parkinson, E.K. Serum metabolomics in animal models and human disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 478–483. [Google Scholar] [CrossRef]
- Savoia, C.; Volpe, M.; Grassi, G.; Borghi, C.; Rosei, E.A.; Touyz, R.M. Personalized medicine—a modern approach for the diagnosis and management of hypertension. Clin. Sci. 2017, 131, 2671–2685. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Shah, S.H.; Corwin, E.J.; Fiehn, O.; Fitzgerald, R.L.; Gerszten, R.E.; Illig, T.; Rhee, E.P.; Srinivas, P.R.; Wang, T.; et al. Potential Impact and Study Considerations of Metabolomics in Cardiovascular Health and Disease: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000032. [Google Scholar] [CrossRef] [Green Version]



| S/N | Study | Methods | Results | References |
|---|---|---|---|---|
| 1 | A targeted metabolomics MRM-MS study on identifying potential hypertension biomarkers in human plasma and evaluating acupuncture effects | Multiple reaction monitoring—mass spectrometry (MRM-MS) of plasma samples from healthy and hypertensive patients | Forty-seven (47) chemical entities were detected in plasma samples of patients. Oleic acid and myoinositol were strongly correlated | [6] Yang et al., 2016 |
| 2 | Central systolic pressure and a nonessential amino acid metabolomics profile: the African Prospective study on the Early Detection and Identification of Cardiovascular disease and Hypertension | NMR spectroscopy, liquid chromatography-tandem mass spectrometry and gas chromatography–time of flight-mass spectrometry of plasma and urine samples | Thirty four metabolites were to be differentiated between Black and White groups. cSBP and cPP inversely correlated with various non-essential amino acids only in Blacks | [9] Mels et al., 2019 |
| 3 | Essential hypertension: A filtered serum-based metabolomics study | NMR metabolomics of filtered serum from 64 essential hypertension (EH) patients and 59 healthy controls (HC) | Alanine, arginine, methionine, pyruvate, adenine, and uracil were found to correctly classify 99% of cases from HC, which also correlated in both isolated elevated DBP as well as combined elevated systolic-diastolic blood pressure | [10] Ameta et al., 2017 |
| 4 | Application of chemometrics to 1H NMR spectroscopic data to investigate a relationship between human serum metabolic profiles and hypertension | 1H NMR spectroscopy of serum profiles of patients with low/normal, borderline and high SBP | The study distinguished low/normal SBP serum samples from borderline and high SBP samples; however, borderline and high SBP samples were not distinguishable from each other. Serum metabolic profiles correlated with SBP, which was attributed to lipoproteins | [15] Brindle et al., 2003 |
| 5 | Effects of four different antihypertensive drugs on plasma metabolomic profiles in patients with essential hypertension | Ultrahigh performance liquid chromatography-mass spectrometry of plasma samples from 313 hypertensive Finnish men | BP decreases correlated with decreases in long-chain acylcanitines (amlodipine and losartan), medium and long-chain FAs (bisoprolol), and an increase in plasma uric acid levels and urea metabolites (hydrochlorothazide) | [20] Hitunen et al., 2017 |
| 6 | Metabolomic study for essential hypertension patients based on dried blood spot mass spectrometry approach | Dried blood spot method coupled with direct infusion mass spectrometry (MS) metabolomic analysis of 87 essential hypertension (EH) patients and 91 healthy controls (HC) | Gly, Orn, C10, Orn/Cit, Phe/Tyr, and C5-OH/C8 were reported to be key metabolites that differentiated EH patients from HC individuals and can be considered biomarkers for hypertension | [31] Bai et al., 2018 |
| 7 | Metabolomic signature of early vascular aging (EVA) in hypertension | Untargeted metabolomic approach of plasma samples of age-, BMI-, and sex-matched groups of EVA (n = 79) and non-EVA (n = 73) individuals with hypertension | Four metabolites lysophosphatidylcholines (LPCs), LPC 18:2, LPC 16:0, LPC 18:0 and LPC 18:1 were associated with EVA. Hypertensive patients with the 4 downregulated LPCs had 3.8 higher risk of EVA compared to those with upregulated LPCs | [32] Polonis et al., 2020 |
| 8 | Global plasma metabolomics to identify potential biomarkers of blood pressure progression | Liquid- and gas-chromatography coupled to mass spectrometry of individuals not on BP-lowering medication at baseline and followed up 5 years later | In the cohort group, ceramide, triacylglycerol, total glycerolipids, oleic acid, and cholesterylester were correlated with DBP change. In the validation cohort, diacylglycerol (36:2) and monoacylglycerol (18:0) were associated with DBP change. | [33] Lin et al., 2020 |
| 9 | Identification of essential hypertension biomarkers in human urine by non-targeted metabolomics based on UPLC-Q-TOF/MS | Ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS) metabolomics of urine samples from 75 cases from each group of EH and HC | Ten potential biomarkers including L-methionine representing amino acid metabolism, fatty acid metabolism, steroid hormone biosynthesis and oxidative stress were found to differentiate between EH and HC groups. | [34] Zhao et al., 2018 |
| 10 | Sexual dimorphism of metabolomic profile in arterial hypertension | Targeted plasma metabolomic profiles of 28 individuals (13 women and 15 men) with essential arterial hypertension and 36 HC (18 women and 18 men) | Twenty-nine metabolites were found to discriminate the metabolic sexual dimorphism of hypertension. These metabolites are related to phospholipidic and cardiac remodeling, arginine/nitric oxide pathway and antihypertensive and insulin resistance mechanisms | [35] Goita et al., 2020 |
| 11 | Metabolomic characterization of hypertension and dyslipidemia | Serum metabolomics of healthy UK population using gas chromatography–mass spectrometry and ultraperformance liquid chromatography–mass spectrometry approach | The study identified 26 and 46 metabolites considered as potential biomarkers of hypertension and dyslipidemia, respectively, which were associated with the metabolisms of fatty acid metabolism, glycerophospholipid metabolism, alanine, aspartate and glutamate | [36] Ke et al., 2018 |
| 12 | An ultrasonication-assisted extraction and derivatization protocol for GC/TOFMS-based metabolite profiling | A gas chromatography/time-of-flight mass spectrometry(GC/TOFMS) of human serum samples EH and HC individuals | Identified metabolite markers that were associated with hypertension to innclude glucosamine, D-sorbitol, 1-stearoylglycerol, and homocysteine | [39] Liu et al., 2011 |
| 13 | Metabolomic heterogeneity of pulmonary arterial hypertension | A combination of high-throughput liquid-and-gas-chromatography-based mass spectrometry metabolomics of human lung tissue from 8 normal and 8 pulmonary arterial hypertension patients | Metabolites revealed disrupted glycolysis, increased TCA cycle, and fatty acid with altered oxidation pathways in the human PAH lung suggesting specific metabolic pathways contributing to increased ATP synthesis responsible for the vascular remodeling process in severe pulmonary hypertension | [61] Zhao et al., 2014 |
| 15 | An untargeted metabolomics study of blood pressure: findings from the Bogalusa Heart Study | Untargeted, ultrahigh performance liquid chromatography-tandem mass spectroscopy metabolomics profiling among 1249 BHS participants | A total of 24 novel metabolites robustly associated with BP including 3 amino acid and nucleotide metabolites, 7 cofactor and vitamin or xenobiotic metabolites and 10 lipid metabolites and their various metabolic pathways | [79] He et al., 2020 |
| 14 | Top-down lipidomics reveals ether lipid deficiency in blood plasma of hypertensive patients | Plasma lipidomics study of 19 hypertensive males and 51 normotensive male controls using top-down shotgun profiling on a LTQ Orbitrap hybrid mass spectrometer | Plasma of hypertensive individuals had decreased content of ether lipids. Ether phosphatidylcholines and ether phosphatidylethanolamines comprising arachidonic (20:4) and docosapentaenoic (22:5) fatty acid moieties, were more diminished as well as content of free cholesterol | [81] Graessler et al., 2009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onuh, J.O.; Qiu, H. Metabolic Profiling and Metabolites Fingerprints in Human Hypertension: Discovery and Potential. Metabolites 2021, 11, 687. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11100687
Onuh JO, Qiu H. Metabolic Profiling and Metabolites Fingerprints in Human Hypertension: Discovery and Potential. Metabolites. 2021; 11(10):687. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11100687
Chicago/Turabian StyleOnuh, John Oloche, and Hongyu Qiu. 2021. "Metabolic Profiling and Metabolites Fingerprints in Human Hypertension: Discovery and Potential" Metabolites 11, no. 10: 687. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11100687