Central and Peripheral Oxygen Distribution in Two Different Modes of Interval Training
Abstract
:1. Introduction
2. Methods
3. Results
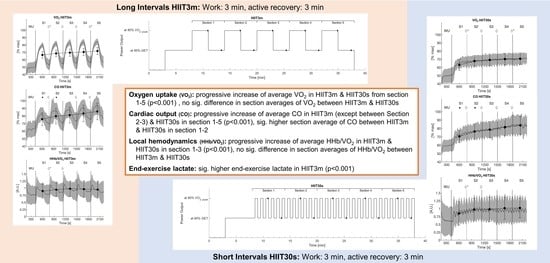
3.1. Aerobic Rate
3.2. Heart Rate
3.3. Cardiac Output
3.4. Stroke Volume
3.5. Muscle Hemodynamics
3.6. End-Exercise Lactate
4. Discussion
4.1. Oxygen Uptake and Central Responses
4.2. Microvascular O2 Provision
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part I: Cardiopulmonary emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Guiraud, T.; Nigam, A.; Juneau, M.; Meyer, P.; Gayda, M.; Bosquet, L. Acute responses to high-intensity intermittent exercise in CHD patients. Med. Sci. Sports Exerc. 2011, 43, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, P.; Guiraud, T.; Gayda, M.; Juneau, M.; Bosquet, L.; Nigam, A. High-intensity aerobic interval training in a patient with stable angina pectoris. Am. J. Phys. Med. Rehabil. 2010, 89, 83–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchheit, M.; Laursen, P.B. High-intensity interval training, solutions to the programming puzzle: Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013, 43, 927–954. [Google Scholar] [CrossRef] [PubMed]
- Helgerud, J.; Høydal, K.; Wang, E.; Karlsen, T.; Berg, P.; Bjerkaas, M.; Simonsen, T.; Helgesen, C.; Hjorth, N.; Bach, R.; et al. Aerobic high-intensity intervals improve VO2max more than moderate training. Med. Sci. Sports Exerc. 2007, 39, 665–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milanović, Z.; Sporis, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Lundby, C. Skeletal muscle vasodilatation during maximal exercise in health and disease. J. Physiol. 2012, 590, 6285–6296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murias, J.M.; Spencer, M.D.; DeLorey, D.S.; Gurd, B.J.; Kowalchuk, J.M.; Paterson, D.H.; Vissing, K.; Latchman, H.; Lamboley, C.; Lamb, G.D.; et al. Speeding of VO2 kinetics during moderate-intensity exercise subsequent to heavy-intensity exercise is associated with improved local O2 distribution. J. Appl. Physiol. 2011, 111, 1410–1415. [Google Scholar] [CrossRef]
- Belfry, G.R.; Paterson, D.H.; Murias, J.M.; Thomas, S.G. The effects of short recovery duration on VO2 and muscle deoxygenation during intermittent exercise. Eur. J. Appl. Physiol. 2012, 112, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-C.; Wang, C.-H.; Lin, P.-S.; Hsu, C.-C.; Cherng, W.-J.; Huang, S.-C.; Liu, M.-H.; Chiang, C.-L.; Wang, J.-S. Aerobic interval training improves oxygen uptake efficiency by enhancing cerebral and muscular hemodynamics in patients with heart failure. Int. J. Cardiol. 2013, 167, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zafeiridis, A.; Kounoupis, A.; Dipla, K.; Kyparos, A.; Nikolaidis, M.G.; Smilios, I.; Vrabas, I.S. Oxygen delivery and muscle deoxygenation during continuous, long- and short-interval exercise. Int. J. Sports Med. 2015, 36, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Sue, D.Y.; Wasserman, K.; Moricca, R.B.; Casaburi, R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease use of the V-slope method for anaerobic threshold determination. Chest 1988, 94, 931–938. [Google Scholar] [CrossRef]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [CrossRef]
- Iannetta, D.; Inglis, E.C.; Mattu, A.T.; Fontana, F.Y.; Pogliaghi, S.; Keir, D.A.; Murias, J.M. A critical evaluation of current methods for exercise prescription in women and men. Med. Sci. Sports Exerc. 2020, 52, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.; Noël-Savina, E.; Prévot, G.; Têtu, L.; Pillard, F.; Riviere, D.; Didier, A. determination of cardiac output in pulmonary hypertension using impedance cardiography. Respir. Int. Rev. Thorac. Dis. 2018, 96, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Moshkovitz, Y.; Kaluski, E.; Milo, O.; Vered, Z.; Cotter, G. Recent developments in cardiac output determination by bioimpedance: Comparison with invasive cardiac output and potential cardiovascular applications. Curr. Opin. Cardiol. 2004, 19, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Richard, R.; Lonsdorfer-Wolf, E.; Charloux, A.; Doutreleau, S.; Buchheit, M.; Oswald-Mammosser, M.; Lampert, E.; Mettauer, B.; Geny, B.; Lonsdorfer, J. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur. J. Appl. Physiol. 2001, 85, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Delpy, D.T.; Cope, M.; Van Der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Van Beekvelt, M.C.P.; Borghuis, M.S.; Van Engelen, B.G.M.; Wevers, R.A.; Colier, W.N.J.M. Adipose tissue thickness affects in vivo quantitative near-IR spectroscopy in human skeletal muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- DeLorey, D.S.; Kowalchuk, J.M.; Paterson, D.H. Effect of age on O(2) uptake kinetics and the adaptation of muscle deoxygenation at the onset of moderate-intensity cycling exercise. J. Appl. Physiol. 2004, 97, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stöcker, F.; Von Oldershausen, C.; Paternoster, F.K.; Schulz, T.; Oberhoffer, R. Relationship of post-exercise muscle oxygenation and duration of cycling exercise. BMC Sports Sci. Med. Rehabil. 2016, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Stocker, F.; Von Oldershausen, C.; Paternoster, F.K.; Schulz, T.; Oberhoffer, R. End-exercise ΔHHb/ΔVO2and post-exercise local oxygen availability in relation to exercise intensity. Clin. Physiol. Funct. Imaging 2017, 37, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murias, J.M.; Kowalchuk, J.M.; Paterson, D.H. Speeding of Vo2 kinetics with endurance training in old and young men is associated with improved matching of local O2 delivery to muscle O2 utilization. J. Appl. Physiol. 2010, 108, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnley, M.; Jones, A.M. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Saltin, B.; Rådegran, G.; Koskolou, M.D.; Roach, R. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol. Scand. 1998, 162, 421–436. [Google Scholar] [CrossRef]
- Gerbino, A.; Ward, S.A.; Whipp, B.J. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J. Appl. Physiol. 1996, 80, 99–107. [Google Scholar] [CrossRef]
- Rossiter, H.; Ward, S.A.; Kowalchuk, J.M.; Howe, F.; Griffiths, J.R.; Whipp, B.J. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J. Physiol. 2001, 537, 291–303. [Google Scholar] [CrossRef]
- Tschakovsky, M.E.; Sheriff, D.D. Immediate exercise hyperemia: Contributions of the muscle pump vs. rapid vasodilation. J. Appl. Physiol. 2004, 97, 739–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Mean ± SD | N |
|---|---|---|
| Age [years] | 24.3 ± 3.6 | 24 |
| Height [cm] | 181.4 ± 5.1 | 24 |
| Weight [kg] | 75.9 ± 7.6 | 24 |
| Skinfold thickness at m. vastus lateralis [mm] | 8.0 ± 3.2 | 24 |
| Peak oxygen uptake (VO2peak) [L*min−1] | 4.11 ± 0.53 | 24 |
| Relative peak oxygen uptake (VO2peak) [mL*min−1 *kg−1] | 54.1 ± 5.3 | 24 |
| Gas Exchange Threshold (GET) [% VO2peak] | 52.9 ± 8.4 | 24 |
| Respiratory Compensation Point (RCP) [% VO2peak] | 82.6 ± 6.9 | 24 |
| peak heart rate (HRpeak) [bpm] | 185.0 ± 7.7 | 24 |
| peak cardiac output (COpeak) [L*min−1] | 25.4 ± 3.4 | 17 |
| peak stroke volume (SVpeak) [ml] | 144.1 ± 19.4 | 17 |
| peak power output (POpeak) [W] | 359.5 ± 44.8 | 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksoll, K.S.H.; Mühlberger, A.; Stöcker, F. Central and Peripheral Oxygen Distribution in Two Different Modes of Interval Training. Metabolites 2021, 11, 790. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110790
Ksoll KSH, Mühlberger A, Stöcker F. Central and Peripheral Oxygen Distribution in Two Different Modes of Interval Training. Metabolites. 2021; 11(11):790. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110790
Chicago/Turabian StyleKsoll, Korbinian Sebastian Hermann, Alexander Mühlberger, and Fabian Stöcker. 2021. "Central and Peripheral Oxygen Distribution in Two Different Modes of Interval Training" Metabolites 11, no. 11: 790. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11110790