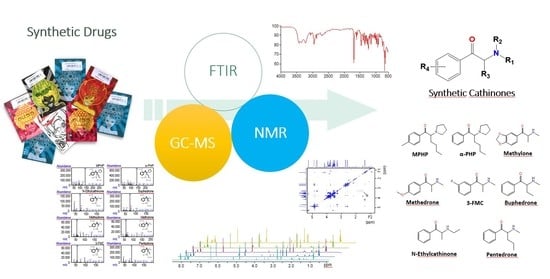
Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques
Abstract
:1. Introduction
2. Results and Discussion
2.1. ATR-FTIR Analysis
2.2. GC-MS Analysis
2.3. NMR Analysis
3. Materials and Methods
3.1. Reagents
3.2. Samples
3.3. ATR-FTIR Analysis
3.4. GC-MS Analysis
3.5. NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Teixeira, H.M.; Câmara, J.S. Synthetic cathinones: An evolving class of new psychoactive substances. Crit. Rev. Toxicol. 2019, 49, 549–566. [Google Scholar] [CrossRef]
- EURAD. Psychoactive Substances: Issues for Policy Makers. Available online: https://www.unodc.org/documents/ungass2016/Contributions/Civil/EURAD/Psychoactive_Substances_Exec_Summary.pdf (accessed on 4 December 2019).
- EMCDDA. European Drug Report 2017: Trends and Developments. Available online: https://www.emcdda.europa.eu/publications/edr/trends-developments/2017_en (accessed on 22 October 2019).
- Gavriilidis, G.; Kyriakoudi, A.; Tiniakos, D.; Rovina, N.; Koutsoukou, A. "Bath Salts" intoxication with multiorgan failure and left-sided ischemic colitis: A case report. Hippokratia 2015, 19, 363–365. [Google Scholar] [PubMed]
- Araújo, A.M.; Valente, M.J.; Carvalho, M.; Dias da Silva, D.; Gaspar, H.; Carvalho, F.; de Lourdes Bastos, M.; Guedes de Pinho, P. Raising awareness of new psychoactive substances: Chemical analysis and in vitro toxicity screening of ‘legal high’ packages containing synthetic cathinones. Arch. Toxicol. 2015, 89, 757–771. [Google Scholar] [CrossRef]
- Meyers, K.; Kaynak, Ö.; Bresani, E.; Curtis, B.; McNamara, A.; Brownfield, K.; Kirby, K.C. The availability and depiction of synthetic cathinones (bath salts) on the Internet: Do online suppliers employ features to maximize purchases? Int. J. Drug Policy 2015, 26, 670–674. [Google Scholar] [CrossRef] [Green Version]
- Valente, M.J.; Guedes de Pinho, P.; Bastos, M.d.L.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Valente, M.J.; Araújo, A.M.; Bastos, M.d.L.; Fernandes, E.; Carvalho, F.; Guedes de Pinho, P.; Carvalho, M. Editor’s Highlight: Characterization of Hepatotoxicity Mechanisms Triggered by Designer Cathinone Drugs (β-Keto Amphetamines). Toxicol. Sci. 2016, 153, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Prosser, J.M.; Nelson, L.S. The Toxicology of Bath Salts: A Review of Synthetic Cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schifano, F.; Papanti, G.D.; Orsolini, L.; Corkery, J.M. Novel psychoactive substances: The pharmacology of stimulants and hallucinogens. Expert Rev. Clin. Pharmacol. 2016, 9, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Calinski, D.M.; Kisor, D.F.; Sprague, J.E. A review of the influence of functional group modifications to the core scaffold of synthetic cathinones on drug pharmacokinetics. Psychopharmacology 2019, 236, 881–890. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Andrzejczak, D. Next generation of novel psychoactive substances on the horizon–A complex problem to face. Drug Alcohol Depend. 2015, 157, 1–17. [Google Scholar] [CrossRef]
- Archer, T.; Kostrzewa, R.M. Synthetic Cathinones: Neurotoxic Health Hazards and Potential for Abuse. In Synthetic Cathinones: Novel Addictive and Stimulatory Psychoactive Substances; Zawilska, J.B., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–10. [Google Scholar]
- Karila, L.; Megarbane, B.; Cottencin, O.; Lejoyeux, M. Synthetic Cathinones: A New Public Health Problem. Curr. Neuropharmacol. 2015, 13, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hout, M.C.; Bingham, T. “A Costly Turn On”: Patterns of use and perceived consequences of mephedrone based head shop products amongst Irish injectors. Int. J. Drug Policy 2012, 23, 188–197. [Google Scholar] [CrossRef]
- Spiller, H.A.; Ryan, M.L.; Weston, R.G.; Jansen, J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin. Toxicol. 2011, 49, 499–505. [Google Scholar] [CrossRef]
- Barrios, L.; Grison-Hernando, H.; Boels, D.; Bouquie, R.; Monteil-Ganiere, C.; Clement, R. Death following ingestion of methylone. Int. J. Leg. Med. 2016, 130, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Giorgetti, R.; Pichini, S.; Pantano, F.; Marinelli, E.; Busardò, F.P. Synthetic cathinones related fatalities: An update. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 268–274. [Google Scholar] [PubMed]
- Romanek, K.; Stenzel, J.; Schmoll, S.; Schrettl, V.; Geith, S.; Eyer, F.; Rabe, C. Synthetic cathinones in Southern Germany–characteristics of users, substance-patterns, co-ingestions, and complications. Clin. Toxicol. 2017, 55, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Palamar, J.J.; Salomone, A.; Vincenti, M.; Cleland, C.M. Detection of “bath salts” and other novel psychoactive substances in hair samples of ecstasy/MDMA/“Molly” users. Drug Alcohol Depend. 2016, 161, 200–205. [Google Scholar] [CrossRef] [Green Version]
- Wojcieszak, J.; Andrzejczak, D.; Wojtas, A.; Gołembiowska, K.; Zawilska, J.B. Effects of the new generation α-pyrrolidinophenones on spontaneous locomotor activities in mice, and on extracellular dopamine and serotonin levels in the mouse striatum. Forensic Toxicol. 2018, 36, 334–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assi, S.; Guirguis, A.; Halsey, S.; Fergus, S.; Stair, J.L. Analysis of ‘legal high’ substances and common adulterants using handheld spectroscopic techniques. Anal. Methods 2015, 7, 736–746. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, M.; Celiński, R.; Kowalska, T.; Sajewicz, M. Fatal case of poisoning with a new cathinone derivative: α-propylaminopentiophenone (N-PP). Forensic Toxicol. 2018, 36, 525–533. [Google Scholar] [CrossRef] [Green Version]
- DRE. Decree Law No. 54/2013 of 17 April 2013, Administrative Rule 154/2013. Available online: https://dre.pt/pesquisa/-/search/260421/details/ (accessed on 24 July 2020).
- Liu, C.; Jia, W.; Li, T.; Hua, Z.; Qian, Z. Identification and analytical characterization of nine synthetic cathinone derivatives N-ethylhexedrone, 4-Cl-pentedrone, 4-Cl-α-EAPP, propylone, N-ethylnorpentylone, 6-MeO-bk-MDMA, α-PiHP, 4-Cl-α-PHP, and 4-F-α-PHP. Drug Test. Anal. 2017, 9, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Sequeira, M.; de Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC–MS. J. Anal. Toxicol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.; Sandgren, V.; Svensson, S.; Konradsson, P.; Dunne, S.; Josefsson, M.; Dahlén, J. Prediction of designer drugs: Synthesis and spectroscopic analysis of synthetic cathinone analogs that may appear on the Swedish drug market. Drug Test. Anal. 2018, 10, 1076–1098. [Google Scholar] [CrossRef] [PubMed]
- Maheux, C.R.; Copeland, C.R. Chemical analysis of two new designer drugs: Buphedrone and pentedrone. Drug Test. Anal. 2012, 4, 17–23. [Google Scholar] [CrossRef]
- Maheux, C.R.; Alarcon, I.Q.; Copeland, C.R.; Cameron, T.S.; Linden, A.; Grossert, J.S. Identification of polymorphism in ethylone hydrochloride: Synthesis and characterization. Drug Test. Anal. 2016, 8, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Westphal, F.; Junge, T.; Girreser, U.; Greibl, W.; Doering, C. Mass, NMR and IR spectroscopic characterization of pentedrone and pentylone and identification of their isocathinone by-products. Forensic Sci. Int. 2012, 217, 157–167. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; Taylor & Francis: New York, NY, USA, 1998. [Google Scholar]
- Kavanagh, P.; O’Brien, J.; Fox, J.; O’Donnell, C.; Christie, R.; Power, J.D.; McDermott, S.D. The analysis of substituted cathinones. Part 3. Synthesis and characterisation of 2,3-methylenedioxy substituted cathinones. Forensic Sci. Int. 2012, 216, 19–28. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., McKelvy, M.L., Eds.; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Zuba, D. Identification of cathinones and other active components of ‘legal highs’ by mass spectrometric methods. Trac Trends Anal. Chem. 2012, 32, 15–30. [Google Scholar] [CrossRef]
- Kohyama, E.; Chikumoto, T.; Tada, H.; Kitaichi, K.; Horiuchi, T.; Ito, T. Differentiation of the Isomers of N-Alkylated Cathinones by GC-EI-MS-MS and LC-PDA. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2016, 32, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Paillet-Loilier, M.; Cesbron, A.; Le Boisselier, R.; Bourgine, J.; Debruyne, D. Emerging drugs of abuse: Current perspectives on substituted cathinones. Subst. Abus. Rehabil. 2014, 5, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Gwak, S.; Arroyo-Mora, L.E.; Almirall, J.R. Qualitative analysis of seized synthetic cannabinoids and synthetic cathinones by gas chromatography triple quadrupole tandem mass spectrometry. Drug Test. Anal. 2015, 7, 121–130. [Google Scholar] [CrossRef]
- Lum, B.J.; Hibbert, D.B.; Brophy, J. Identification of Substituted Cathinones (β-keto phenethylamines) by Heptafluorobutyric Anhydride (HFBA) Chemical Derivatization and Gas Chromatography Mass Spectrometry. Swafs J. 2013, 34, 7–30. [Google Scholar]
- Moldoveanu, S.C.; David, V. Derivatization Methods in GC and GC/MS. In Gas Chromatography-Derivatization, Sample Preparation, Application; Kusch, P., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Kumazawa, T.; Hara, K.; Hasegawa, C.; Uchigasaki, S.; Lee, X.-P.; Seno, H.; Suzuki, O.; Sato, K. Fragmentation Pathways of Trifluoroacetyl Derivatives of Methamphetamine, Amphetamine, and Methylenedioxyphenylalkylamine Designer Drugs by Gas Chromatography/Mass Spectrometry. Int. J. Spectrosc. 2011, 2011, 318148. [Google Scholar] [CrossRef] [Green Version]
- Alsenedi, K.A.; Morrison, C. Comparison of six derivatizing agents for the determination of nine synthetic cathinones using gas chromatography-mass spectrometry. Anal. Methods 2017, 9, 2732–2743. [Google Scholar] [CrossRef] [Green Version]
- Ash, J.; Hickey, L.; Goodpaster, J. Formation and identification of novel derivatives of primary amine and zwitterionic drugs. Forensic Chem. 2018, 10, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Westphal, F.; Junge, T.; Rösner, P.; Fritschi, G.; Klein, B.; Girreser, U. Mass spectral and NMR spectral data of two new designer drugs with an α-aminophenone structure: 4′-Methyl-α-pyrrolidinohexanophenone and 4′-methyl-α-pyrrolidinobutyrophenone. Forensic Sci. Int. 2007, 169, 32–42. [Google Scholar] [CrossRef]
- Majchrzak, M.; Rojkiewicz, M.; Celiński, R.; Kuś, P.; Sajewicz, M. Identification and characterization of new designer drug 4-fluoro-PV9 and α-PHP in the seized materials. Forensic Toxicol. 2016, 34, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, N.; Shimokawa, Y.; Kawamura, M.; Kikura-Hanajiri, R.; Hakamatsuka, T. Chemical analysis of a benzofuran derivative, 2-(2-ethylaminopropyl)benzofuran (2-EAPB), eight synthetic cannabinoids, five cathinone derivatives, and five other designer drugs newly detected in illegal products. Forensic Toxicol. 2014, 32, 266–281. [Google Scholar] [CrossRef]
- Balci, M. 13C Chemical Shifts of Organic Compounds; Elsevier Science: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Souza, L.F.; Vieira, T.S.; Alcantara, G.B.; Lião, L.M. HR-MAS NMR for Rapid Identification of Illicit Substances in Tablets and Blotter Papers Seized by Police Department. J. Braz. Chem. Soc. 2016, 27, 2141–2148. [Google Scholar] [CrossRef]
- Maheux, C.R.; Copeland, C.R.; Pollard, M.M. Characterization of Three Methcathinone Analogs: 4-Methylmethcathinone, Methylone, and bk-MBDB. Microgram J. 2010, 7, 42–49. [Google Scholar]
- Zancajo, V.M.R.; Brito, J.; Carrasco, M.P.; Bronze, M.R.; Moreira, R.; Lopes, A. Analytical profiles of “legal highs” containing cathinones available in the area of Lisbon, Portugal. Forensic Sci. Int. 2014, 244, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.; Kusz, J.; Książek, M.; Pieprzyca, E.; Rojkiewicz, M. Spectroscopic characterization and crystal structures of two cathinone derivatives: N-ethyl-2-amino-1-phenylpropan-1-one (ethcathinone) hydrochloride and N-ethyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-CEC) hydrochloride. Forensic Toxicol. 2017, 35, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, R.P. Fluoromethcathinone, a new substance of abuse. Forensic Sci. Int. 2009, 185, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, A.; Girotto, S.; Berti, B.; Stair, J.L. Identification of new psychoactive substances (NPS) using handheld Raman spectroscopy employing both 785 and 1064nm laser sources. Forensic Sci. Int. 2017, 273, 113–123. [Google Scholar] [CrossRef] [Green Version]









| Sample Number | Product Name | Description | Appearance | Quantity (g) | Composition Indicated on the Label |
|---|---|---|---|---|---|
| 1 | Unknown | n.a. 1 | White powder | 1 | n.a. 1 |
| 2 | Flakka | n.a. 1 | Brownish crystal | 5 | n.a. 1 |
| 3 | Bloom | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 4 | Bloom | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 5 | Bloom | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 6 | Bloom | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 7 | Bloom | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 8 | Charlie | Plant feeder | Light yellow powder | 1 | 100% Ketones |
| 9 | Bliss | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| 10 | Bliss | Plant feeder | White tablets | 5 tablets | Per Pill: 120 mg lactose, 20 mg magnesium stearate, 100 mg corn starch, 160 mg ketones, 50 mg calcium stearate, 4 mg E142, 6 mg E132, 20 mg E124 |
| 11 | Blast | Plant feeder | White powder | 1 | 89% Ketones, 10% caffeine, 1% glucose |
| 12 | Kick | Plant feeder | White powder | 1 | 94% Ketones, 5% caffeine, 1% glucose |
| Peak No. | Compound Name | Direct Analysis | Derivatization with TFAA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RT (min) | MF | MW | Ions (m/z) | RT (min) | MF | MW | Ions (m/z) | ||
| 1 | MPHP 1 | 18.654 | C17H25NO | 259 | 140, 91, 119, 41 | - | - | - | - |
| 2 | α-PHP 1 | 17.950 | C17H25NO | 245 | 140, 77, 96, 105 | - | - | - | - |
| 3 | N-Ethylcathinone | 10.658 | C11H15NO | 177 | 72, 44, 77, 105 | 12.173 | C13H14F3NO2 | 273 | 168, 105, 140, 77 |
| 4 | Buphedrone | 10.785 | C11H15NO | 177 | 72, 77, 44, 105 | 11.813 | C13H14F3NO2 | 273 | 168, 105, 77, 110 |
| 5 | Methedrone | 14.820 | C11H15NO2 | 193 | 58, 135, 77, 92 | 16.096 | C13H14F3NO3 | 289 | 135, 77, 154, 92 |
| 6 | Ethylphenidate | 17.357 | C14H19NO2 | 233 | 84, 91, 56, 164 | 18.538 | C17H20F3NO3 | 343 | 180, 164, 67, 55 |
| 7 | Caffeine 1 | 17.775 | C8H10N4O2 | 194 | 194, 109, 67, 55 | - | - | - | - |
| 8 | Methylone | 16.314 | C11H13NO3 | 207 | 58, 149, 65, 121 | 17.239 | C13H12F3NO4 | 303 | 149, 154, 121, 110 |
| 9 | 3-FMC | 10.007 | C10H12FNO | 181 | 58, 95, 75, 123 | 10.837 | C12H11F4NO2 | 277 | 154, 110, 123, 95 |
| 10 | Isopentedrone | 11.516 | C12H17NO | 191 | 120, 42, 118, 91 | 13.239 | C14H16F3NO2 | 287 | 110, 216, 182, 140 |
| 11 | Pentedrone | 12.083 | C12H17NO | 191 | 86, 44, 77, 105 | 13.098 | C14H16F3NO2 | 287 | 182, 140, 105, 77 |
| Position |  |  |  |  | ||||
| MPHP | α-PHP | Methylone | Methedrone | |||||
| 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | |
| 1 | 197.9 | - | 198.4 | - | 195.9 | - | 196.4 | - |
| 2 | 69.9 | 5.27, 1H, t, J = 5.06 Hz | 70.0 | 5.31, 1H, t, J = 5.06 Hz | 59.9 | 5.06–5.01, 1H, q, J = 7.23 Hz | 59.9 | 5.11–5.05, 1H, q, J = 7.21 Hz |
| 3 | 30.2 | 2.18–2.01, 2H, m | 30.0 | 2.18–2.01, 2H, m | 16.3 | 1.64, 3H, d, J = 7.24 Hz | 16.2 | 1.64, 3H, d, J = 7.24 Hz |
| 4 | 25.8 | 1.28–1.22, 1H, m 1.15–1.11, 1H, m | 25.3 | 1.28–1.18, 1H, m 1.15–1.11, 1H, m | - | - | - | - |
| 5 | 22.4 | 1.28–1.21, 2H, m | 22.3 | 1.28–1.18, 2H, m | - | - | - | - |
| 6 | 13.3 | 0.76, 3H, t, J = 6.92 Hz | 13.2 | 0.76, 3H, t, J = 7.02 Hz | - | - | - | - |
| 1′ | 131.6 | - | 134.1 | - | 127.3 | - | 125.8 | - |
| 2′ | 129.7 | 7.96, 2H, d, J = 8.16 Hz | 129.5 | 8.06, 2H, d, J = 7.52 Hz | 108.5 | 7.47, 1H, d, J = 1.64 Hz | 132.2 | 8.04, 2H, at, J = 8.84 Hz |
| 3′ | 130.6 | 7.47, 2H, d, J = 8.08 Hz | 129.9 | 7.65, 2H, t, J = 7.82 Hz | 148.9 | - | 115.1 | 7.14, 2H, d, J = 8.96 Hz |
| 4′ | 148.4 | - | 136.3 | 7.81, 1H, t, J = 7.46 Hz | 154.1 | - | 165.4 | - |
| 5′ | 130.6 | 7.47, 2H, d, J = 8.08 Hz | 129.9 | 7.65, 2H, t, J = 7.82 Hz | 109.1 | 7.04, 1H, d, J = 8.28 Hz | 115.1 | 7.14, 2H, d, J = 8.96 Hz |
| 6′ | 129.7 | 7.96, 2H, d, J = 8.16 Hz | 129.5 | 8.06, 2H, d, J = 7.52 Hz | 127.0 | 7.70–7.68, 1H, dd, J = 8.32, 1.72 Hz | 132.2 | 8.04, 2H, at, J = 8.84 Hz |
| 7′ | 21.6 | 2.46, 3H, s | - | - | 103.2 | 6.14, 2H, s | 56.3 | 3.48, 3H, s |
| 1″ | 52.6 | 3.80–3.69, 1H, m 3.39–3.32, 1H, m | 52.6 | 3.80–3.70, 1H, m 3.40–3.32, 1H, m | 31.5 | 2.82, 3H, s | 31.6 | 2.83, 3H, s |
| 2″ | 23.3 | 2.18–2.01, 2H, m | 23.3 | 2.18–2.01, 2H, m | - | - | - | - |
| 3″ | 23.4 | 2.30–2.23, 1H, m 2.18–2.01, 1H, m | 23.4 | 2.27–2.21, 1H, m 2.18–2.01, 1H, m | - | - | - | - |
| 4″ | 55.8 | 3.80–3.69, 1H, m 3.10–3.04, 1H, m | 55.8 | 3.80–3.70, 1H, m 3.12–3.06, 1H, m | - | - | - | - |
| Position |  |  |  |  | |||||
| N-Ethylcathinone | Buphedrone | Pentedrone | 3-Fluoromethcathinone | ||||||
| 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | 19F (δ/ppm) | 13C (δ/ppm) | 1H (δ/ppm, protons, multiplicity a, coupling constants) | |
| 1 | 198.2 | - | 197.8 | - | 197.8 | - | 196.9, d, J = 2.15 Hz | - | |
| 2 | 58.6 | 5.22–5.16, 1H, m | 65.0 | 5.22–5.16, 1H, m | 64.2 | 5.19, 1H, t, J = 5.26 Hz | 60.4 | 5.14–5.09, 1H, q, J = 7.28 Hz | |
| 3 | 16.1 | 1.63, 3H, d, J = 7.4 Hz | 23.6 | 2.24–2.05, 2H, m | 32.4 | 2.12–1.95, 2H, m | 15.7 | 1.65, 3H, d, J = 7.28 Hz | |
| 4 | - | - | 7.9 | 0.89, 3H, t, J = 7.58 Hz | 17.6 | 1.41–1.32, 1H, m 1.29–1.17, 1H, m | - | - | |
| 5 | - | - | - | - | 13.4 | 0.86, 3H, t, J = 7.28 Hz | - | - | |
| 1′ | 132.9 | - | 133.7 | - | 136.0 | - | 134.9, d, J = 6.75 Hz | - | |
| 2′ | 129.5 | 8.06, 2H, d, J = 8.20 Hz | 129.4 | 8.06, 2H, d, J = 8.20 Hz | 129.4 | 8.05, 2H, d, J = 7.40 Hz | 115.9, d, J = 23.0 Hz | 7.86, 1H, d, J = 7.76 | |
| 3′ | 129.9 | 7.65, 2H, at, J = 7.62 Hz | 129.9 | 7.65, 2H, at, J = 7.62 Hz | 129.8 | 7.64, 2H, t, J = 7.82 Hz | −114.3 | 162.0, d, J = 244.8 Hz | - |
| 4′ | 136.0 | 7.81, 1H, at, J = 7.44 Hz | 136.1 | 7.81, 1H, at J = 7.44 Hz | 133.6 | 7.80, 1H, t, J = 7.48 Hz | 122.8, d, J = 21.4 Hz | 7.53, 1H, dt, J = 8.30, 2.13Hz | |
| 5′ | 129.9 | 7.65, 2H, at, J = 7.62 Hz | 129.9 | 7.65, 2H, at, J = 7.62 Hz | 129.8 | 7.64, 2H, t, J = 7.82 Hz | 131.8, d, J = 7.94 Hz | 7.68–7.62, 1H, m | |
| 6′ | 129.5 | 8.06, 2H, d, J = 8.20 Hz | 129.4 | 8.06, 2H, d, J = 8.20 Hz | 129.4 | 8.05, 2H, d, J = 7.40 Hz | 125.6, d, J = 2.89 Hz | 7.78, 1H, d, J = 9.28 Hz | |
| 1″ | 41.9 | 3.31–3.22, 1H, m 3.22–3.13, 1H, m | 32.2 | 2.82, 3H, s | 32.3 | 2.81, 3H, s | 31.5 | 2.85, 3H, s | |
| 2″ | 11.3 | 1.39, 3H, t, J = 7.32 Hz | - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.L.; Alves, V.L.; Aguiar, J.; Caldeira, M.J.; Teixeira, H.M.; Câmara, J.S. Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques. Metabolites 2021, 11, 144. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11030144
Gonçalves JL, Alves VL, Aguiar J, Caldeira MJ, Teixeira HM, Câmara JS. Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques. Metabolites. 2021; 11(3):144. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11030144
Chicago/Turabian StyleGonçalves, João L., Vera L. Alves, Joselin Aguiar, Maria J. Caldeira, Helena M. Teixeira, and José S. Câmara. 2021. "Structure Assignment of Seized Products Containing Cathinone Derivatives Using High Resolution Analytical Techniques" Metabolites 11, no. 3: 144. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11030144