Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap
Abstract
:1. From Bariatric to Metabolic Surgery—A Name Change or a Game-Changer?
2. The Metabolome as a Field of Studying the Effects of Bariatric and Metabolic Surgery
3. Bariatric and Metabolic Surgery in the Era of Artificial Intelligence
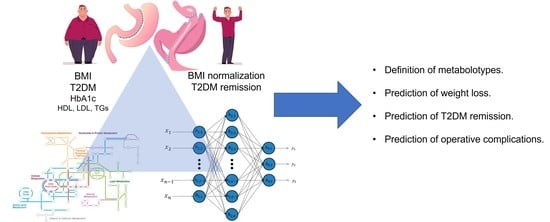
4. Deep Learning and Metabolomics—Too Hard to Handle or the Coming of an Era?
- (1)
- Lack of human-centric data availability: Bariatric patients constitute a surgical population that is submitted to vigorous follow-up. Most importantly, there are two large international bariatric databases (IFSO® Registry and MBSAQIP® Database) that are regularly updated. Starting to integrate metabolic profiles of patients before and at discrete follow-up visits after BMS apart from demographic, clinical, routine laboratory, and nutrition data would serve as a scaffold for a population-wide metabolomics database.
- (2)
- Dimensionality and overfitting: This limitation is generated by the asymmetrical distribution of low numbers of samples and too many measured features in the context of metabolomics (high-dimension low-sample size data; HDLSS). Again, the key to this could be found in the large populations of existing bariatric databases, provided they start to integrate data on metabolites.
- (3)
- Lack of metabolomics-specific DL features: This problem is non-BMS specific, but it is rather ubiquitous for metabolomics. In contradistinction to genomics and proteomics, metabolomics still lacks well-defined problem statements and methods. Relevant studies in the aforementioned fields employ strategies that convert DNA/RNA sequences and protein structures (alpha-helix, beta-strand, loop region) into encoded representations that are suitable for convoluted neural network (CNN) applications [33]. More advanced is the conversion of nonimage data to image-like data suitable for processing with CNN [36].
- (4)
- Challenging model validation: Given that metabolomics datasets are considered HDLSS, one measure to overcome the current lack of large populations would be to apply nonconventional validation methods. For example, a widely adopted validation method, k-fold cross validation, leads to biased performance with small sample sizes. On the contrary, nested cross-validation has shown stable performance regardless of the sample size [37]. Metabolomics studies on MBS patients with limited sample sizes could benefit from this approach.
- (1)
- Basic science: Existing studies on retrieving metabolites and their pathophysiological role could benefit from a layered structure of analysis after applying the strategies mentioned earlier. A good relevant example is the study of Date and Kikuchi [35]: their experimental model was yellowfin goby (Acanthogobius flavimanus), their objective was metabolic characterization depending on geographical distribution, the sample type was muscle tissue, they studied two sets of metabolites (water-soluble components, n = 170; methanol-soluble components, n = 1022), and the utilized DL algorithm was a deep neural network analytical approach that yielded better classification accuracy as compared to ML algorithms. Analogous experiments could be implemented to existing animal models of BMS [38,39] with the purpose of metabolomic characterization before and after initial operation and monitoring of BMS effectiveness at a second level.
- (2)
- Safety (complications): DL algorithms could be implemented to map the metabolome of BMS candidates and to correlate specific metabolotypes with certain adverse outcomes, such as defective wound healing that may lead to a leak or to susceptibility to the formation of adhesions.
- (3)
- Effectiveness (bariatric outcomes): Weight loss is the main objective of the vast majority of BMS. Consequently, being able to predict in advance which patient is going to benefit from which operation based on their metabolomic synthesis would be of utmost research and clinical interest. DL could be implemented in order to yield data from existing populations and through hidden layers that reveal favorable and avoidable metabolomic setups.
- (4)
- Associated medical problems (including T2DM): Existing evidence focuses on the metabolic aspects of metabolomics on patients who undergo BMS [22,29]. The advent of DL, following the implementation of strategies to overcome limitations, could contribute to a more widespread performance of such studies.
Funding
Conflicts of Interest
References
- Ramos, A.; Kow, L.; Brown, W.; Welbourn, R.; Dixon, J.; Kinsman, R.; Walton, P. The IFSO Global Registry, 5th IFSO Global Registry Report. 2019. Available online: https://www.ifso.com/pdf/5th-ifso-global-registry-report-september-2019.pdf (accessed on 15 March 2022).
- Bhandari, M.; Fobi, M.A.L.; Buchwald, J.N. Standardization of Bariatric Metabolic Procedures: World Consensus Meeting Statement. Obes. Surg. 2019, 29, 309–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, P.R.; Kashyap, S.R.; Wolski, K.; Brethauer, S.A.; Kirwan, J.P.; Pothier, C.E.; Thomas, S.; Abood, B.; Nissen, S.E.; Bhatt, D.L. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N. Engl. J. Med. 2012, 366, 1567–1576. Available online: https://pubmed.ncbi.nlm.nih.gov/22449319/ (accessed on 14 May 2022). [CrossRef] [PubMed]
- Schauer, P.R.; Bhatt, D.L.; Kirwan, J.P.; Wolski, K.; Aminian, A.; Brethauer, S.A.; Navaneethan, S.D.; Singh, R.P.; Pothier, C.E.; Nissen, S.E.; et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes—5-Year Outcomes. N. Engl. J. Med. 2017, 376, 641–651. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1600869 (accessed on 14 May 2022). [CrossRef] [PubMed] [Green Version]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Nanni, G.; Castagneto, M.; Bornstein, S.; Rubino, F. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 Year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015, 386, 964–973. Available online: http://www.thelancet.com/article/S0140673615000756/fulltext (accessed on 27 September 2020). [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Capristo, E.; Chamseddine, G.; Bornstein, S.R.; Rubino, F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021, 397, 293–304. Available online: http://www.thelancet.com/article/S0140673620326490/fulltext (accessed on 14 May 2022). [CrossRef]
- Buchwald, H. The Evolution of Metabolic/Bariatric Surgery. Obes. Surg. 2014, 24, 1126–1135. Available online: https://pubmed.ncbi.nlm.nih.gov/25008469/ (accessed on 15 March 2022). [CrossRef]
- Rubino, F. From Bariatric to Metabolic Surgery: Definition of a New Discipline and Implications for Clinical Practice. Curr. Atheroscler. Rep. 2013, 15, 369. Available online: https://pubmed.ncbi.nlm.nih.gov/24194467/ (accessed on 16 August 2020). [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.M.M.; Zimmet, P.Z.; del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. Available online: https://pubmed.ncbi.nlm.nih.gov/27222544/ (accessed on 15 March 2022). [CrossRef] [Green Version]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Nissen, S.E.; Kattan, M.W. Predicting 10-year risk of end-organ complications of type 2 diabetes with and without metabolic surgery: A machine learning approach. Diabetes Care 2020, 43, 852–859. Available online: https://pubmed.ncbi.nlm.nih.gov/32029638/ (accessed on 20 April 2021). [CrossRef]
- Samczuk, P.; Ciborowski, M.; Kretowski, A. Application of Metabolomics to Study Effects of Bariatric Surgery. J. Diabetes Res. 2018, 2018, 6270875. Available online: https://pubmed.ncbi.nlm.nih.gov/29713650/ (accessed on 15 March 2022). [CrossRef] [Green Version]
- Ha, J.; Jang, M.; Kwon, Y.-K.; Park, Y.S.; Park, D.J.; Lee, J.-H.; Lee, H.-J.; Ha, T.K.; Kim, Y.-J.; Han, S.-M.; et al. Metabolomic Profiles Predict Diabetes Remission after Bariatric Surgery. J. Clin. Med. 2020, 9, 3897. Available online: https://pubmed.ncbi.nlm.nih.gov/33271740/ (accessed on 15 March 2022). [CrossRef] [PubMed]
- Vaz, M.; Pereira, S.S.; Monteiro, M.P. Metabolomic signatures after bariatric surgery—A systematic review. Rev. Endocr. Metab. Disord. 2021, 1–17. Available online: https://pubmed.ncbi.nlm.nih.gov/34855133/ (accessed on 15 March 2022).
- Ha, J.; Kwon, Y.; Park, S. Metabolomics in Bariatric Surgery: Towards Identification of Mechanisms and Biomarkers of Metabolic Outcomes. Obes. Surg. 2021, 31, 4564–4574. Available online: https://pubmed.ncbi.nlm.nih.gov/34318371/ (accessed on 15 March 2022). [CrossRef] [PubMed]
- Ceperuelo-Mallafre, V.; Llaurado, G.; Keiran, N.; Benaiges, E.; Astiarraga, B.; Martinez, L.; Pellitero, S.; González-Clemente, J.M.; Rodríguez, A.; Fernández-Real, J.M.; et al. Preoperative circulating succinate levels as a biomarker for diabetes remission after bariatric surgery. Diabetes Care 2019, 42, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.E.; Fadnes, D.J.; Røst, T.H.; Pedersen, E.R.; Andersen, J.R.; Vage, V.; Ulvik, A.; Midttun, Ø.; Ueland, P.M.; Nygård, O.K.; et al. Inflammatory markers, the tryptophan-kynurenine pathway, and vitamin B status after bariatric surgery. PLoS ONE 2018, 13, e0192169. Available online: https://pubmed.ncbi.nlm.nih.gov/29401505/ (accessed on 15 March 2022). [CrossRef] [Green Version]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Metabolomic Analysis of the Improvements in Insulin Secretion and Resistance After Sleeve Gastrectomy: Implications of the Novel Biomarkers. Obes. Surg. 2020, 31, 43–52. Available online: https://pubmed.ncbi.nlm.nih.gov/32815103/ (accessed on 15 March 2022). [CrossRef]
- Luo, P.; Yu, H.; Zhao, X.; Bao, Y.; Hong, C.S.; Zhang, P.; Tu, Y.; Yin, P.; Gao, P.; Wei, L.; et al. Metabolomics Study of Roux-en-Y Gastric Bypass Surgery (RYGB) to Treat Type 2 Diabetes Patients Based on Ultraperformance Liquid Chromatography–Mass Spectrometry. J. Proteome Res. 2016, 15, 1288–1299. Available online: https://pubmed.ncbi.nlm.nih.gov/26889720/ (accessed on 15 March 2022). [CrossRef]
- Zhao, L.; Ni, Y.; Yu, H.; Zhang, P.; Zhao, A.; Bao, Y.; Liu, J.; Chen, T.; Xie, G.; Panee, J.; et al. Serum stearic acid/palmitic acid ratio as a potential predictor of diabetes remission after Roux-en-Y gastric bypass in obesity. FASEB J. 2016, 31, 1449–1460. Available online: https://pubmed.ncbi.nlm.nih.gov/28007782/ (accessed on 15 March 2022). [CrossRef] [Green Version]
- Kwon, Y.; Jang, M.; Lee, Y.; Ha, J.; Park, S. Amino Acid Metabolites and Slow Weight Loss in the Early Postoperative Period after Sleeve Gastrectomy. J. Clin. Med. 2020, 9, 2348. Available online: https://pubmed.ncbi.nlm.nih.gov/32717870/ (accessed on 15 March 2022). [CrossRef]
- Abidi, W.; Nestoridi, E.; Feldman, H.; Stefater, M.; Clish, C.; Thompson, C.C.; Stylopoulos, N. Differential Metabolomic Signatures in Patients with Weight Regain and Sustained Weight Loss After Gastric Bypass Surgery: A Pilot Study. Am. J. Dig. Dis. 2019, 65, 1144–1154. Available online: https://pubmed.ncbi.nlm.nih.gov/31385097/ (accessed on 15 March 2022). [CrossRef]
- Palau-Rodriguez, M.; Tulipani, S.; Marco-Ramell, A.; Miñarro, A.; Jauregui, O.; Gonzalez-Dominguez, R.; Sanchez-Pla, A.; Ramos-Molina, B.; Tinahones, F.J.; Andres-Lacueva, C. Characterization of Metabolomic Profile Associated with Metabolic Improvement after Bariatric Surgery in Subjects with Morbid Obesity. J. Proteome Res. 2018, 17, 2704–2714. Available online: https://pubmed.ncbi.nlm.nih.gov/29893570/ (accessed on 2 April 2022). [CrossRef]
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. Available online: https://pmc/articles/PMC5507106/ (accessed on 15 May 2022). [CrossRef] [PubMed]
- Duchnowski, P.; Hryniewiecki, T.; Kuśmierczyk, M.; Szymański, P. The usefulness of selected biomarkers in patients with valve disease. Biomarkers Med. 2018, 12, 1341–1346. Available online: https://0-www-futuremedicine-com.brum.beds.ac.uk/doi/full/10.2217/bmm-2018-0101 (accessed on 15 May 2022). [CrossRef] [PubMed] [Green Version]
- Hui, S.; Cowan, A.J.; Zeng, X.; Yang, L.; TeSlaa, T.; Li, X.; Bartman, C.; Zhang, Z.; Jang, C.; Wang, L.; et al. Quantitative Fluxomics of Circulating Metabolites. Cell Metab. 2020, 32, 676–688.e4. Available online: https://pubmed.ncbi.nlm.nih.gov/32791100/ (accessed on 15 May 2022). [CrossRef] [PubMed]
- Loftus, T.J.; Tighe, P.J.; Filiberto, A.C.; Efron, P.A.; Brakenridge, S.C.; Mohr, A.M.; Rashidi, P.; Upchurch, G.R., Jr.; Bihorac, A. Artificial Intelligence and Surgical Decision-Making. JAMA Surg. 2020, 155, 148–158. Available online: http://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed/31825465 (accessed on 14 January 2020). [CrossRef]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. Available online: https://pubmed.ncbi.nlm.nih.gov/31015651/ (accessed on 26 February 2022). [CrossRef]
- Pantelis, A.G.; Stravodimos, G.K.; Lapatsanis, D.P. A Scoping Review of Artificial Intelligence and Machine Learning in Bariatric and Metabolic Surgery: Current Status and Future Perspectives. Obes. Surg. 2021, 31, 4555–4563. Available online: https://pubmed.ncbi.nlm.nih.gov/34264433/ (accessed on 15 March 2022). [CrossRef]
- Narath, S.H.; Mautner, S.; Svehlikova, E.; Schultes, B.; Pieber, T.R.; Sinner, F.M.; Gander, E.; Libiseller, G.; Schimek, M.G.; Sourij, H.; et al. An untargeted metabolomics approach to characterize short-term and long-term metabolic changes after bariatric surgery. PLoS ONE 2016, 11, e0161425. Available online: https://pubmed.ncbi.nlm.nih.gov/27584017/ (accessed on 20 April 2021).
- Candi, E.; Tesauro, M.; Cardillo, C.; Di Daniele, N.; Melino, G. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochem. J. 2018, 475, 1019–1035. Available online: https://pubmed.ncbi.nlm.nih.gov/29437994/ (accessed on 20 April 2021). [CrossRef]
- Perakakis, N.; Polyzos, S.A.; Yazdani, A.; Sala-Vila, A.; Kountouras, J.; Anastasilakis, A.D.; Mantzoros, C.S. Non-invasive diagnosis of non-alcoholic steatohepatitis and fibrosis with the use of omics and supervised learning: A proof of concept study. Metabolism 2019, 101, 154005. Available online: https://pubmed.ncbi.nlm.nih.gov/31711876/ (accessed on 15 March 2022). [CrossRef]
- Castañé, H.; Baiges-gaya, G.; Hernández-aguilera, A.; Rodríguez-tomàs, E.; Fernández-arroyo, S.; Herrero, P.; Delpino-Rius, A.; Canela, N.; Menendez, J.A.; Camps, J. Coupling Machine Learning and Lipidomics as a Tool to Investigate Metabolic Dysfunction-Associated Fatty Liver Disease. A General Overview. Biomolecules 2021, 11, 473. Available online: https://pubmed.ncbi.nlm.nih.gov/33810079/ (accessed on 15 March 2022). [CrossRef]
- Pomyen, Y.; Wanichthanarak, K.; Poungsombat, P.; Fahrmann, J.; Grapov, D.; Khoomrung, S. Deep metabolome: Applications of deep learning in metabolomics. Comput. Struct. Biotechnol. J. 2020, 18, 2818–2825. Available online: https://pubmed.ncbi.nlm.nih.gov/33133423/ (accessed on 2 April 2022). [CrossRef] [PubMed]
- Sen, P.; Lamichhane, S.; Mathema, V.B.; McGlinchey, A.; Dickens, A.M.; Khoomrung, S.; Orešič, M. Deep learning meets metabolomics: A methodological perspective. Briefings Bioinform. 2020, 22, 1531–1542. Available online: https://pubmed.ncbi.nlm.nih.gov/32940335/ (accessed on 15 March 2022). [CrossRef] [PubMed]
- Date, Y.; Kikuchi, J. Application of a Deep Neural Network to Metabolomics Studies and Its Performance in Determining Important Variables. Anal. Chem. 2018, 90, 1805–1810. Available online: https://pubmed.ncbi.nlm.nih.gov/29278490/ (accessed on 15 March 2022). [CrossRef] [PubMed]
- Sharma, A.; Vans, E.; Shigemizu, D.; Boroevich, K.A.; Tsunoda, T. DeepInsight: A methodology to transform a non-image data to an image for convolution neural network architecture. Sci. Rep. 2019, 9, 11399. Available online: https://pubmed.ncbi.nlm.nih.gov/31388036/ (accessed on 2 April 2022). [CrossRef] [Green Version]
- Vabalas, A.; Gowen, E.; Poliakoff, E.; Casson, A.J. Machine learning algorithm validation with a limited sample size. PLoS ONE 2019, 14, e0224365. Available online: https://pubmed.ncbi.nlm.nih.gov/31697686/ (accessed on 2 April 2022). [CrossRef]
- Rao, R.S.; Rao, V.; Kini, S. Animal models in bariatric surgery—A review of the surgical techniques and postsurgical physiology. Obes. Surg. 2010, 20, 1293–1305. Available online: https://pubmed.ncbi.nlm.nih.gov/20383602/ (accessed on 23 April 2022). [CrossRef]
- Ashrafian, H.; Bueter, M.; Ahmed, K.; Suliman, A.; Bloom, S.R.; Darzi, A.; Athanasiou, T. Metabolic surgery: An evolution through bariatric animal models. Obes. Rev. 2010, 11, 907–920. Available online: https://pubmed.ncbi.nlm.nih.gov/20051020/ (accessed on 23 April 2022). [CrossRef]
- Gowda, G.A.N.; Gowda, Y.N.; Raftery, D. Expanding the limits of human blood metabolite quantitation using NMR spectroscopy. Anal. Chem. 2014, 87, 706–715. Available online: https://0-pubs-acs-org.brum.beds.ac.uk/doi/full/10.1021/ac503651e (accessed on 23 April 2022). [CrossRef] [Green Version]
- Yin, P.; Lehmann, R.; Xu, G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem. 2015, 407, 4879–4892. Available online: https://pmc/articles/PMC4471316/ (accessed on 23 April 2022). [CrossRef] [Green Version]
- Van Olden, C.C.; Van de Laar, A.W.; Meijnikman, A.S.; Aydin, O.; Van Olst, N.; Hoozemans, J.B.; de Brauw, L.M.; Bruin, S.C.; Acherman, Y.I.Z.; Verheij, J.; et al. A systems biology approach to understand gut microbiota and host metabolism in morbid obesity: Design of the BARIA Longitudinal Cohort Study. J. Intern. Med. 2021, 289, 340–354. Available online: https://pubmed.ncbi.nlm.nih.gov/32640105/ (accessed on 23 April 2022). [CrossRef]

| Type of AI Algorithm | Purpose | Examples |
|---|---|---|
| Supervised machine learning | Classification (categorical output, i.e., obese, not obese, T2DM remission-nonremission) or Regression (continuous output, i.e., weight, BMI, HbA1c level). | Decision trees, random forest, knn, logistic regression |
| Unsupervised machine learning | Clustering (inherent grouping in data, i.e., grouping responders of bariatric surgery based on their metabolomic setup) or Association (discovering the rules that describe large portions of data). | K-means for clustering, a priori algorithms |
| Deep learning | Input and output are connected in layers with relationships that resemble neural networks in the nervous system. These relationships are usually “hidden”. | Convolutional neural networks, artificial neural networks, Bayesian networks. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantelis, A.G. Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap. Metabolites 2022, 12, 458. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050458
Pantelis AG. Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap. Metabolites. 2022; 12(5):458. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050458
Chicago/Turabian StylePantelis, Athanasios G. 2022. "Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap" Metabolites 12, no. 5: 458. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12050458