Shotgun Lipidomics for Differential Diagnosis of HPV-Associated Cervix Transformation
Abstract
:1. Introduction
2. Results
2.1. Clinical Data
2.2. HPV Typing
2.3. mRNA Expression during HPV Infection
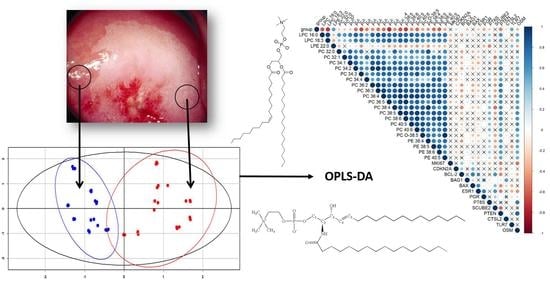
2.4. Cervical Tissue Lipidomics
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Study Design
4.3. Morphological Investigation
4.4. HPV Typing
4.5. mRNA Expression Analysis
4.6. Tissue Preparation for Lipidome Analysis
4.7. Mass Spectrometric Analysis of Lipid Extracts
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World—Summary Report 22 October 2021; ICO HPV Information Centre: Barcelona, Spain, 2021. [Google Scholar]
- Muñoz, N.; Bosch, F.X.; De Sanjosé, S.; Herrero, R.; Castellsagué, X.; Shah, K.V.; Snijders, P.J.F.; Meijer, C.J.L.M. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.X.; Du, H.; Liu, Z.H.; Zhang, L.J.; Wang, C.; Wu, R.F. Evaluation of CIN2+/CIN3+ risk of different HPV subtypes infection combined with abnormal cytology status. Chin. J. Oncol. 2018, 40, 232–238. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins—Gynecology; American College of Obstetricians and Gynecologists. Practice Bulletin No. 157: Cervical Cancer Screening and Prevention. Obstet. Gynecol. 2016, 127, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, M.; Smith, J.H.F.; Tidy, J.A.; Palmer, J.E. Conservative management of CIN2: National Audit of British Society for Colposcopy and Cervical Pathology members’ opinion. J. Obstet. Gynaecol. 2018, 38, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Barut, M.U.; Kale, A.; Kuyumcuoğlu, U.; Bozkurt, M.; Ağaçayak, E.; Özekinci, S.; Gul, T. Analysis of sensitivity, specificity, and positive and negative predictive values of smear and colposcopy in diagnosis of premalignant and malignant cervical lesions. Med. Sci. Monit. 2015, 21, 3860–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beerman, H.; van Dorst, E.B.L.; Kuenen-Boumeester, V.; Hogendoorn, P.C.W. Superior performance of liquid-based versus conventional cytology in a population-based cervical cancer screening program. Gynecol. Oncol. 2009, 112, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Koliopoulos, G.; Nyaga, V.N.; Santesso, N.; Bryant, A.; Martin-Hirsch, P.P.L.; Mustafa, R.A.; Schünemann, H.; Paraskevaidis, E.; Arbyn, M. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst. Rev. 2017, 2017, CD008587. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef]
- Burk, R.D.; Chen, Z.; Saller, C.; Tarvin, K.; Carvalho, A.L.; Scapulatempo-Neto, C.; Silveira, H.C.; Fregnani, J.H.; Creighton, C.J.; Anderson, M.L.; et al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Gromski, P.S.; Muhamadali, H.; Ellis, D.I.; Xu, Y.; Correa, E.; Turner, M.L.; Goodacre, R. A tutorial review: Metabolomics and partial least squares-discriminant analysis—A marriage of convenience or a shotgun wedding. Anal. Chim. Acta 2015, 879, 10–23. [Google Scholar] [CrossRef]
- Porcari, A.M.; Negrão, F.; Tripodi, G.L.; Pitta, D.R.; Campos, E.A.; Montis, D.M.; Martins, A.M.A.; Eberlin, M.N.; Derchain, S.F.M. Molecular Signatures of High-Grade Cervical Lesions. Front. Oncol. 2018, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Coffey, M.J.; Torretti, B.; Mancuso, P. Adipokines and Cysteinyl Leukotrienes in the Pathogenesis of Asthma. J. Allergy 2015, 2015, 157919. [Google Scholar] [CrossRef] [Green Version]
- Vauzour, D.; Martinsen, A.; Layé, S. Neuroinflammatory processes in cognitive disorders: Is there a role for flavonoids and n-3 polyunsaturated fatty acids in counteracting their detrimental effects? Neurochem. Int. 2015, 89, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; DiVittore, N.A.; Young, M.M.; Jia, Z.; Xie, K.; Ritty, T.M.; Kester, M.; Fox, T.E. Altered sphingolipid metabolism in patients with metastatic pancreatic cancer. Biomolecules 2013, 3, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Lee, A.; Park, Y.S.; Lee, S.C.; Park, S.Y.; Han, S.Y.; Kim, K.P.; Kim, Y.H.; Yoo, C.W.; Kim, H.K. Alteration in lipid and protein profiles of ovarian cancer similarity to breast cancer. Int. J. Gynecol. Cancer 2011, 21, 1566–1572. [Google Scholar] [CrossRef]
- Goto, T.; Terada, N.; Inoue, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Sumiyoshi, S.; Kobayashi, T.; et al. The expression profile of phosphatidylinositol in high spatial resolution imaging mass spectrometry as a potential biomarker for prostate cancer. PLoS ONE 2014, 9, e90242. [Google Scholar] [CrossRef] [Green Version]
- Nekrasova, M.E.; Chagovets, V.V.; Starodubtseva, N.L.; Kononikhin, A.S.; Salimova, D.F.; Tokareva, A.O.; Lagutin, V.V.; Naumov, V.A.; Nazarova, N.M.; Frankevich, V.E.; et al. LIPID markers of cervical epithelium neoplastic transformation in HPV-associated diseases. Akush. Ginekol. Obstet. Gynecol. 2018, 4, 64–70. [Google Scholar] [CrossRef]
- Sans, M.; Gharpure, K.; Tibshirani, R.; Zhang, J.; Liang, L.; Liu, J.; Young, J.H.; Dood, R.L.; Sood, A.K.; Eberlin, L.S. Metabolic markers and statistical prediction of serous ovarian cancer aggressiveness by ambient ionization mass spectrometry imaging. Cancer Res. 2017, 77, 2903–2913. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, Y.; Guan, L.; Zhang, H.; Sun, J.; Gong, X.; Li, D.; Chen, P.; Ma, Z.; Liang, X.; et al. Discovery of phosphatidic acid, phosphatidylcholine, and phosphatidylserine as biomarkers for early diagnosis of endometriosis. Front. Physiol. 2018, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Adamyan, L.V.; Starodubtseva, N.; Borisova, A.; Stepanian, A.A.; Chagovets, V.; Salimova, D.; Wang, Z.; Kononikhin, A.; Popov, I.; Bugrova, A.; et al. Direct Mass Spectrometry Differentiation of Ectopic and Eutopic Endometrium in Patients with Endometriosis. J. Minim. Invasive Gynecol. 2018, 25, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Chagovets, V.; Wang, Z.; Kononikhin, A.; Starodubtseva, N.; Borisova, A.; Salimova, D.; Popov, I.; Kozachenko, A.; Chingin, K.; Chen, H.; et al. A Comparison of Tissue Spray and Lipid Extract Direct Injection Electrospray Ionization Mass Spectrometry for the Differentiation of Eutopic and Ectopic Endometrial Tissues. J. Am. Soc. Mass Spectrom. 2018, 29, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Chagovets, V.; Kononikhin, A.; Starodubtseva, N.; Kostyukevich, Y.; Popov, I.; Frankevich, V.; Nikolaev, E. Peculiarities of data interpretation upon direct tissue analysis by Fourier transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. 2016, 22, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, A.O.; Chagovets, V.V.; Starodubtseva, N.L.; Nazarova, N.M.; Nekrasova, M.E.; Kononikhin, A.S.; Frankevich, V.E.; Nikolaev, E.N.; Sukhikh, G.T. Feature selection for OPLS discriminant analysis of cancer tissue lipidomics data. J. Mass Spectrom. 2020, 55, e4457. [Google Scholar] [CrossRef]
- Sukhikh, G.; Chagovets, V.; Wang, X.; Rodionov, V.; Kometova, V.; Tokareva, A.; Kononikhin, A.; Starodubtseva, N.; Chingin, K.; Chen, H.; et al. Combination of low-temperature electrosurgical unit and extractive electrospray ionization mass spectrometry for molecular profiling and classification of tissues. Molecules 2019, 24, 2957. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, S.; Tateya, I.; Hayasaka, T.; Masaki, N.; Takizawa, Y.; Ohno, S.; Kojima, T.; Kitani, Y.; Kitamura, M.; Hirano, S.; et al. Increased expression of phosphatidylcholine (16:0/18:1) and (16:0/18:2) in thyroid papillary cancer. PLoS ONE 2012, 7, e48873. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.Y.; Choi, S.H.; Park, Y.S.; Park, D.Y.; Park, Y.I.; Hwang, I.; Ryu, M.H.; Kwon, C.H.; Lee, J.H.; Bang, G.; et al. Lipid MALDI MS profiles of gastric cancer. Open Proteom. J. 2014, 7, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.C.; Lee, J.H.; Bang, G.; Choi, S.H.; Kim, Y.H.; Kim, K.P.; Kim, H.K.; Ro, J. Lipid profiles for HER2-positive breast cancer. Anticancer Res. 2013, 33, 2467–2472. [Google Scholar]
- Zhao, X.; Brusadelli, M.G.; Sauter, S.; Kovacic, M.B.; Zhang, W.; Romick-Rosendale, L.E.; Lambert, P.F.; Setchell, K.D.R.; Wells, S.I. Lipidomic profiling links the fanconi anemia pathway to glycosphingolipid metabolism in head and neck cancer cells. Clin. Cancer Res. 2018, 24, 2700–2709. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.C.; Bang, G.; Lee, J.H.; Kim, K.P.; Kim, Y.H.; Kim, H.K.; Chung, J. Erratum: Low C24-OH and C22-OH sulfatides in human renal cell carcinoma. J. Mass Spectrom. 2016, 51, 182. [Google Scholar] [CrossRef] [Green Version]
- Morita, Y.; Sakaguchi, T.; Ikegami, K.; Goto-Inoue, N.; Hayasaka, T.; Hang, V.T.; Tanaka, H.; Harada, T.; Shibasaki, Y.; Suzuki, A.; et al. Lysophosphatidylcholine acyltransferase 1 altered phospholipid composition and regulated hepatoma progression. J. Hepatol. 2013, 59, 292–299. [Google Scholar] [CrossRef]
- Altadill, T.; Dowdy, T.M.; Gill, K.; Reques, A.; Menon, S.S.; Moiola, C.P.; Lopez-Gil, C.; Coll, E.; Matias-Guiu, X.; Cabrera, S.; et al. Metabolomic and Lipidomic Profiling Identifies the Role of the RNA Editing Pathway in Endometrial Carcinogenesis. Sci. Rep. 2017, 7, 8803. [Google Scholar] [CrossRef]
- Marien, E.; Meister, M.; Muley, T.; Fieuws, S.; Bordel, S.; Derua, R.; Spraggins, J.; Van De Plas, R.; Dehairs, J.; Wouters, J.; et al. Non-small cell lung cancer is characterized by dramatic changes in phospholipid profiles. Int. J. Cancer 2015, 137, 1539–1548. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, F.; Wang, F.; Li, C.; Meng, G.; Duan, H.; Ma, Q.; Zhang, W. Serum peptidome profiling analysis for the identification of potential biomarkers in cervical intraepithelial neoplasia patients. Biochem. Biophys. Res. Commun. 2015, 465, 476–480. [Google Scholar] [CrossRef]
- Woo, H.M.; Kim, K.M.; Choi, M.H.; Jung, B.H.; Lee, J.; Kong, G.; Nam, S.J.; Kim, S.; Bai, S.W.; Chung, B.C. Mass spectrometry based metabolomic approaches in urinary biomarker study of women’s cancers. Clin. Chim. Acta 2009, 400, 63–69. [Google Scholar] [CrossRef]
- Hasim, A.; Ali, M.; Mamtimin, B.; Ma, J.-Q.; Li, Q.-Z.; Abudula, A. Metabonomic signature analysis of cervical carcinoma and precancerous lesions in women by 1H NMR spectroscopy. Exp. Ther. Med. 2012, 3, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xu, J.; Zhang, R.; Shen, G.; Song, Y.; Sun, J.; He, J.; Zhan, Q.; Abliz, Z. Assessment of data pre-processing methods for LC-MS/MS-based metabolomics of uterine cervix cancer. Analyst 2013, 138, 2669–2677. [Google Scholar] [CrossRef]
- Yin, M.Z.; Tan, S.; Li, X.; Hou, Y.; Cao, G.; Li, K.; Kou, J.; Lou, G. Identification of phosphatidylcholine and lysophosphatidylcholine as novel biomarkers for cervical cancers in a prospective cohort study. Tumor Biol. 2016, 37, 5485–5492. [Google Scholar] [CrossRef]
- Yang, K.; Xia, B.; Wang, W.; Cheng, J.; Yin, M.; Xie, H.; Li, J.; Ma, L.; Yang, C.; Li, A.; et al. A Comprehensive Analysis of Metabolomics and Transcriptomics in Cervical Cancer. Sci. Rep. 2017, 7, 43353. [Google Scholar] [CrossRef]
- Walker, H.; Burrell, M.; Flatley, J.; Powers, H. A metabolite profiling method for diagnosis of precancerous cervical lesions and HPV persistence. Bioanalysis 2017, 9, 601–608. [Google Scholar] [CrossRef]
- Tzafetas, M.; Mitra, A.; Paraskevaidi, M.; Bodai, Z.; Kalliala, I.; Bowden, S.; Lathouras, K.; Rosini, F.; Szasz, M.; Savage, A.; et al. The intelligent knife (iKnife) and its intraoperative diagnostic advantage for the treatment of cervical disease. Proc. Natl. Acad. Sci. USA 2020, 117, 7338–7346. [Google Scholar] [CrossRef] [Green Version]
- Paraskevaidi, M.; Cameron, S.J.S.; Whelan, E.; Bowden, S.; Tzafetas, M.; Mitra, A.; Semertzidou, A.; Athanasiou, A.; Bennett, P.R.; MacIntyre, D.A.; et al. Laser-assisted rapid evaporative ionisation mass spectrometry (LA-REIMS) as a metabolomics platform in cervical cancer screening. EBioMedicine 2020, 60, 103017. [Google Scholar] [CrossRef]
- Del Pino, M.; Svanholm-Barrie, C.; Torné, A.; Marimon, L.; Gaber, J.; Sagasta, A.; Persing, D.H.; Ordi, J. MRNA biomarker detection in liquid-based cytology: A new approach in the prevention of cervical cancer. Mod. Pathol. 2015, 28, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Ikenberg, H.; Bergeron, C.; Schmidt, D.; Griesser, H.; Alameda, F.; Angeloni, C.; Bogers, J.; Dachez, R.; Denton, K.; Hariri, J.; et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: Results of the PALMS study. J. Natl. Cancer Inst. 2013, 105, 1550–1557. [Google Scholar] [CrossRef]
- Den Boon, J.A.; Pyeon, D.; Wang, S.S.; Horswill, M.; Schiffman, M.; Sherman, M.; Zuna, R.E.; Wang, Z.; Hewitt, S.M.; Pearson, R.; et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc. Natl. Acad. Sci. USA 2015, 112, E3255–E3264. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Tian, H.; Wang, L. Effects of PTEN on the proliferation and apoptosis of colorectal cancer cells via the phosphoinositol-3-kinase/Akt pathway. Oncol. Rep. 2015, 33, 1828–1836. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.X.; Cao, L.Y.; Chen, X.; Xiao, J.; Zou, Y.; Chen, Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT Pathway in Lung Adenocarcinoma A549 Cells. BioMed Res. Int. 2016, 2016, 2476842. [Google Scholar] [CrossRef] [Green Version]
- Lax, S.F. Pathology of Endometrial Carcinoma. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2017; pp. 75–96. ISBN 9783319431376. [Google Scholar]
- Peng, L.N.; Shi, W.T.; Feng, H.R.; Wei, C.Y.; Yin, Q.N. Effect of miR-301a/PTEN pathway on the proliferation and apoptosis of cervical cancer. Innate Immun. 2019, 25, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Coleman, N. Oncostatin M receptor is a novel therapeutic target in cervical squamous cell carcinoma. J. Pathol. 2014, 232, 386–390. [Google Scholar] [CrossRef] [Green Version]
- Kucia-Tran, J.A.; Tulkki, V.; Smith, S.; Scarpini, C.G.; Hughes, K.; Araujo, A.M.; Yan, K.Y.M.; Botthof, J.; Pérez-Gómez, E.; Quintanilla, M.; et al. Overexpression of the oncostatin-M receptor in cervical squamous cell carcinoma is associated with epithelial-mesenchymal transition and poor overall survival. Br. J. Cancer 2016, 115, 212–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadducci, A.; Guerrieri, M.E.; Greco, C. Tissue biomarkers as prognostic variables of cervical cancer. Crit. Rev. Oncol. Hematol. 2013, 86, 104–129. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.W.; Kim, S.W.; Kim, S.; Kim, J.H.; Cho, N.H.; Kim, J.W.; Kim, Y.T. Prevalence and clinical relevance of cyclooxygenase-1 and -2 expression in stage IIB cervical adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, G.E.; Pyo, H.R.; Cho, N.H.; Keum, K.C.; Lee, C.G.; Seong, J.; Suh, C.O.; Park, T.K. Differential Cyclooxygenase-2 Expression in Squamous Cell Carcinoma and Adenocarcinoma of the Uterine Cervix. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 822–829. [Google Scholar] [CrossRef]
- Bourmenskaya, O.; Shubina, E.; Trofimov, D.; Rebrikov, D.; Sabdulaeva, E.; Nepsha, O.; Bozhenko, V.; Rogovskaya, S.; Sukhikh, G. Host gene expression profiling of cervical smear is eligible for cancer risk evaluation. J. Clin. Pathol. 2013, 66, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Swierczynski, J.; Hebanowska, A.; Sledzinski, T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J. Gastroenterol. 2014, 20, 2279–2303. [Google Scholar] [CrossRef] [PubMed]
- Podo, F.; Sardanelli, F.; Iorio, E.; Canese, R.; Carpinelli, G.; Fausto, A.; Canevari, S. Abnormal Choline Phospholipid Metabolism in Breast and Ovary Cancer: Molecular Bases for Noninvasive Imaging Approaches. Curr. Med. Imaging Rev. 2007, 3, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Podo, F.; Canevari, S.; Canese, R.; Pisanu, M.E.; Ricci, A.; Iorio, E. Tumour Phospholipid Metabolism. Exp. Oncol. 2011, 19, 413–439. [Google Scholar] [CrossRef]
- Dória, M.L.; Cotrim, Z.; MacEdo, B.; Simões, C.; Domingues, P.; Helguero, L.; Domingues, M.R. Lipidomic approach to identify patterns in phospholipid profiles and define class differences in mammary epithelial and breast cancer cells. Breast Cancer Res. Treat. 2012, 133, 635–648. [Google Scholar] [CrossRef]
- Rysman, E.; Brusselmans, K.; Scheys, K.; Timmermans, L.; Derua, R.; Munck, S.; Van Veldhoven, P.P.; Waltregny, D.; Daniëls, V.W.; Machiels, J.; et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010, 70, 8117–8126. [Google Scholar] [CrossRef] [Green Version]
- Burch, T.C.; Isaac, G.; Booher, C.L.; Rhim, J.S.; Rainville, P.; Langridge, J.; Baker, A.; Nyalwidhe, J.O. Comparative metabolomic and lipidomic analysis of phenotype stratified prostate cells. PLoS ONE 2015, 10, e0134206. [Google Scholar] [CrossRef] [Green Version]
- Ollila, S.; Hyvönen, M.T.; Vattulainen, I. Polyunsaturation in lipid membranes: Dynamic properties and lateral pressure profiles. J. Phys. Chem. B 2007, 111, 3139–3150. [Google Scholar] [CrossRef]
- Park, Y.S.; Yoo, C.W.; Lee, S.C.; Park, S.J.; Oh, J.H.; Yoo, B.C.; Paik, S.S.; Lee, K.G.; Jin, S.Y.; Kim, S.C.; et al. Lipid profiles for intrahepatic cholangiocarcinoma identified using matrix-assisted laser desorption/ionization mass spectrometry. Clin. Chim. Acta 2011, 412, 1978–1982. [Google Scholar] [CrossRef]
- Krasny, L.; Hoffmann, F.; Ernst, G.; Trede, D.; Alexandrov, T.; Havlicek, V.; Guntinas-Lichius, O.; Von Eggeling, F.; Crecelius, A.C. Spatial segmentation of MALDI FT-ICR MSI Data: A powerful tool to explore the head and neck tumor in situ lipidome. J. Am. Soc. Mass Spectrom. 2014, 26, 36–43. [Google Scholar] [CrossRef]
- Cífková, E.; Holčapek, M.; Lísa, M.; Vrána, D.; Gatěk, J.; Melichar, B. Determination of lipidomic differences between human breast cancer and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. Anal. Bioanal. Chem. 2015, 407, 991–1002. [Google Scholar] [CrossRef]
- Ryu, J.; Bang, G.; Lee, J.H.; Choi, S.H.; Jung, Y.S.; Kim, K.P.; Kim, Y.H.; Kim, H.K. Lipid MALDI MS profiling accurately distinguishes papillary thyroid carcinoma from normal tissue. J. Proteom. Bioinform. 2013, 6, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Terada, N.; Inoue, T.; Kobayashi, T.; Nakayama, K.; Okada, Y.; Yoshikawa, T.; Miyazaki, Y.; Uegaki, M.; Utsunomiya, N.; et al. Decreased expression of lysophosphatidylcholine (16:0/OH) in high resolution imaging mass spectrometry independently predicts biochemical recurrence after surgical treatment for prostate cancer. Prostate 2015, 75, 1821–1830. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [Green Version]
- American Society for Colposcopy and Cervical Pathology Algorithms. 2012 Updated Consensus Guidelines for Managing Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract Dis. 2013, 17, S1–S27. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007, 35, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebisch, G.; Vizcaíno, J.A.; Köfeler, H.; Trötzmüller, M.; Griffiths, W.J.; Schmitz, G.; Spener, F.; Wakelam, M.J.O. Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 2013, 54, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Spearman, C. “Footrule” for measuring correlation. Br. J. Psychol. 1906, 2, 89–108. [Google Scholar] [CrossRef]





| Parameter | ChC (n = 30) | LSIL (n = 30) | HSIL (n = 30) | SCC (n = 20) |
|---|---|---|---|---|
| Age, years | 29 ± 3.7 | 32 ± 4.8 | 34 ± 3.2 | 37 ± 3.3 |
| Height, cm | 167.6 ± 3.9 | 167.2 ± 3.8 | 167.4 ± 4.5 | 166.4 ± 5.8 |
| Body mass, kg | 62.3 ± 7.0 | 63.1 ± 10.5 | 63.3 ± 11.6 | 63.6 ± 11.2 |
| Menarche, years | 12.9 ± 1.0 | 13.1 ± 1.3 | 12.8 ± 1.1 | 13.2 ± 0.9 |
| Menstrual cycle length, days | 29.2 ± 2.4 | 28.4 ± 1.8 | 28.7 ± 2.1 | 27.7 ± 3.5 |
| Duration of menstruation, days | 5.2 ± 0.8 | 5.4 ± 1.1 | 5.3 ± 0.9 | 5.4 ± 0.5 |
| Number of pregnancies | 45 (21%) | 39 (18%) | 56 (27%) | 73 (34%) |
| Number of spontaneous births | 23 (23%) | 23 (23%) | 25 (25%) | 30 (29%) |
| Number of induced abortions | 9 (12%) | 7 (9%) | 28 (36%) | 33 (43%) |
| Cytological Examination | ChC, n = 30 | LSIL, n = 30 | HSIL, n = 30 | SCC, n = 20 |
|---|---|---|---|---|
| NILM | 6 (20%) | 3 (10%) | 1 (3.3%) | 1 (5%) |
| Chronic cervicitis | 11 (37%) | 6 (20%) | 1 (3.3%) | 1 (5%) |
| ASCUS | 7 (23%) | 6 (20%) | 2 (7%) | - |
| LSIL | 4 (13.4%) | 12 (40%) | 4 (13.4%) | - |
| HSIL | 1 (3.3%) | 3 (10%) | 22 (73%) | 3 (15%) |
| SCC | 1 (3.3%) | - | - | 15 (75%) |
| HPV Groups for for Carcinogenicity | HPV Phylogenetic Group | HPV Type | ChC, n = 30 | LSIL, n = 30 | HSIL, n = 30 | SCC, n = 20 | Total, n = 110 |
|---|---|---|---|---|---|---|---|
| 1 | A9 | 16 | 5 (16.7%) | 9 (30%) | 21 (70%) | 11 (55%) | 46 (42%) |
| 52 | 2 (6.7%) | 4 (13.4%) | - | - | 6 (5.4%) | ||
| 33 | - | 1 (3.3%) | 5 (16.7%) | 2 (10%) | 8 (7.3%) | ||
| 58 | 2 (6.7%) | 4 (13.4%) | 1 (3.3%) | - | 7 (6.4%) | ||
| 31 | 2 (6.7%) | 3 (10%) | 2 (7%) | 2 (10%) | 9 (8.2%) | ||
| 35 | 2 (6.7%) | 1 (3.3%) | 4 (13.4%) | 2 (10%) | 9 (8.2%) | ||
| 2A | A7 | 68 | 1 (3.3%) | 1 (3.3%) | - | 1 (5%) | 3 (2.7%) |
| 1 | A7 | 45 | 2 (6.7%) | - | 2 (7%) | 1 (5%) | 5 (4.5%) |
| 18 | 3 (10%) | 2 (7%) | - | 4 (20%) | 9 (8.2%) | ||
| 59 | - | 1 (3.3%) | 1 (3.3%) | - | 2 (1.8%) | ||
| 39 | 2 (6.7%) | - | - | - | 2 (1.8%) | ||
| 2B | A6 | 66 | - | - | 2 (7%) | 1 (5%) | 3 (2.7%) |
| 1 | 56 | 4 (13.3%) | 3 (10%) | 3 (10%) | - | 10 (9%) | |
| 2B | 53 | 1 (3.3%) | 1 (3.3%) | - | 1 (5%) | 3 (2.7%) | |
| LR | A10 | 6 | - | - | - | 1 (5%) | 1 (0.9%) |
| LR | 44 (55) | 1 (3.3%) | 3 (10%) | 2 (7%) | 2 (10%) | 8 (7.3%) | |
| 2B | A5 | 82 | - | 2 (7%) | 1 (3.3%) | - | 3 (2.7%) |
| 1 | 51 | 3 (10%) | - | 5 (16.7%) | - | 8 (7.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtseva, N.L.; Chagovets, V.V.; Nekrasova, M.E.; Nazarova, N.M.; Tokareva, A.O.; Bourmenskaya, O.V.; Attoeva, D.I.; Kukaev, E.N.; Trofimov, D.Y.; Frankevich, V.E.; et al. Shotgun Lipidomics for Differential Diagnosis of HPV-Associated Cervix Transformation. Metabolites 2022, 12, 503. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12060503
Starodubtseva NL, Chagovets VV, Nekrasova ME, Nazarova NM, Tokareva AO, Bourmenskaya OV, Attoeva DI, Kukaev EN, Trofimov DY, Frankevich VE, et al. Shotgun Lipidomics for Differential Diagnosis of HPV-Associated Cervix Transformation. Metabolites. 2022; 12(6):503. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12060503
Chicago/Turabian StyleStarodubtseva, Natalia L., Vitaliy V. Chagovets, Maria E. Nekrasova, Niso M. Nazarova, Alisa O. Tokareva, Olga V. Bourmenskaya, Djamilja I. Attoeva, Eugenii N. Kukaev, Dmitriy Y. Trofimov, Vladimir E. Frankevich, and et al. 2022. "Shotgun Lipidomics for Differential Diagnosis of HPV-Associated Cervix Transformation" Metabolites 12, no. 6: 503. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo12060503