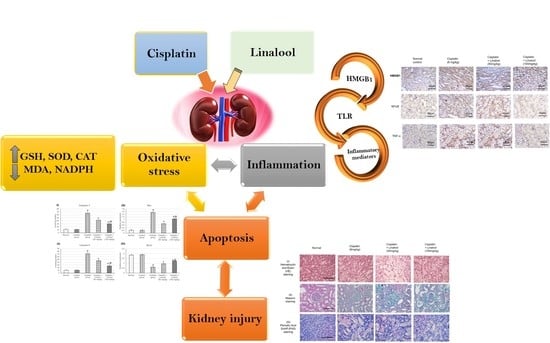
Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Kits
2.2. Animals
2.3. Experimental Design
2.4. Blood and Tissue Samples Collection
2.5. Assessment of Renal Function
2.6. Assessment of Oxidative Stress Markers
2.7. Assessment of Nuclear Factor E2-Related Factor 2 (Nrf2) and Heme Oxygenase-1 (HO-1)
2.8. Assessment of Toll-Like Receptor Pathway Gene Expression
2.9. Assessment of the Toll-Like Receptor Pathway Protein Expressions
2.10. Assessment of Inflammatory Mediators
2.11. Assessment of Apoptotic Markers
2.12. Histopathological Investigation and Immunohistochemical Protein Assay
2.13. Cell Lines Cytotoxicity Assay
2.14. Statistical Analysis
3. Results
3.1. Renal Function
3.2. Oxidative Stress Markers
3.3. Nrf2/HO-1 Signaling Pathway
3.4. TLR Pathway Gene and Protein Expressions
3.5. Inflammatory Mediators
3.6. Apoptotic Markers
3.7. Histopathological Investigation
3.8. Immunohistochemical Protein Assay
3.9. Cytotoxic Activity in HeLa and PC3 Human Cancer Cell Lines
4. Discussion
4.1. Cisplatin and Oxidative Stress in Renal Tissues
4.2. Linalool Attenuation of Cisplatin-Induced Oxidative Stress in Renal Tissues
4.3. The Nrf2 Mediated Oxidative Stress Pathway
4.4. TLRs and Their Tailoring and Ligand Markers
4.5. Amelioration of Inflammatory Mediators
4.6. Linalool and Apoptosis
4.7. Linalool Effect on Cisplatin Potential as A Cytotoxic Agent
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gunaseelan, S.; Balupillai, A.; Govindasamy, K.; Ramasamy, K.; Muthusamy, G.; Shanmugam, M.; Thangaiyan, R.; Robert, B.M.; Prasad Nagarajan, R.; Ponniresan, V.K.; et al. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS ONE 2017, 12, e0176699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, Y.; Hara, C.; Aoki, T.; Sugimoto, N.; Masujima, T. Odor Distinctiveness between Enantiomers of Linalool: Difference in Perception and Responses Elicited by Sensory Test and Forehead Surface Potential Wave Measurement. Chem. Senses 2000, 25, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozek, T.; Tabanca, N.; Demirci, F.; Wedge, D.; Baser, K.H.C. Enantiomeric Distribution of Some Linalool Containing Essential Oils and Their Biological Activities. Rec. Nat. Prod. 2010, 4, 180–192. [Google Scholar]
- Chanotiya, C.; Yadav, A. Enantiomeric Composition of (3R)-(−)- and (3S)-(+)-Linalool in Various Essential Oils of Indian Origin by Enantioselective Capillary Gas Chromatography-Flame Ionization and Mass Spectrometry Detection Methods. Nat. Prod. Commun. 2009, 4, 563–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lucena, J.D.; Gadelha-Filho, C.V.J.; Da Costa, R.O.; De Araújo, D.P.; Lima, F.A.V.; Neves, K.R.T.; Viana, G.S.D.B. L-linalool exerts a neuroprotective action on hemiparkinsonian rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Oner, Z.; Altinoz, E.; Elbe, H.; Ekinci, N. The protective and therapeutic effects of linalool against doxorubicin-induced cardiotoxicity in Wistar albino rats. Hum. Exp. Toxicol. 2019, 38, 803–813. [Google Scholar] [CrossRef]
- Altınok-Yipel, F.; Tekeli, I.O.; Özsoy Şule, Y.; Güvenç, M.; Kaya, A.; Yipel, M. Hepatoprotective Activity of Linalool in Rats Against Liver Injury Induced by Carbon Tetrachloride. Int. J. Vitam. Nutr. Res. 2020, 90, 302–308. [Google Scholar] [CrossRef]
- Peana, A.T.; Marzocco, S.; Popolo, A.; Pinto, A. (-)-Linalool inhibits in vitro NO formation: Probable involvement in the antinociceptive activity of this monoterpene compound. Life Sci. 2006, 78, 719–723. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.M.; Min, J.H.; Kwon, O.K.; Park, M.H.; Park, J.W.; Ahn, H.I.; Hwang, J.Y.; Oh, S.R.; Lee, J.W.; et al. Anti-inflammatory effects of linalool on ovalbumin-induced pulmonary inflammation. Int. Immunopharmacol. 2019, 74, 105706. [Google Scholar] [CrossRef]
- Lee, S.C.; Wang, S.Y.; Li, C.C.; Liu, C.T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018, 26, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Tekeli, I.O.; Atessahin, A.; Sakin, F.; Aslan, A.; Çeribaşı, S.; Yipel, M. Protective effects of conventional and colon-targeted lycopene and linalool on ulcerative colitis induced by acetic acid in rats. Inflammopharmacology 2018, 27, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, G. Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. JBUON 2019, 24, 323–328. [Google Scholar]
- Rodenak-Kladniew, B.; Castro, A.; Starkel, P.; De Saeger, C.; Garcia de Bravo, M.; Crespo, R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018, 199, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepa, B.; Venkatraman Anuradha, C. Effects of linalool on inflammation, matrix accumulation and podocyte loss in kidney of streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Perse, M.; Veceric-Haler, Z. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. Bio. Med. Res. Int. 2018, 2018, 1462802. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, M.; Mou, J.; Zhao, Z.; Yang, J.; Zhu, F.; Pei, G.; Zhu, H.; Wang, Y.; Xu, G.; et al. Pretreatment of Huaiqihuang extractum protects against cisplatin-induced nephrotoxicity. Sci. Rep. 2018, 8, 7333. [Google Scholar] [CrossRef]
- Reeves, W.B. Innate immunity in nephrotoxic acute kidney injury. Trans. Am. Clin. Climatol. Assoc. 2019, 130, 33–40. [Google Scholar] [PubMed]
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.N.; Wang, X.W.; Li, L.Y.; Xu, Z.W.; Huang, H.Y.; Zhao, J.S.; Zhang, D.; Yin, X.; Sheng, J.; Tang, J.T. Pu-erh tea powder preventive effects on cisplatin-induced liver oxidative damage in Wistar rats. Asian Pacific J. Cancer Prev. APJCP 2014, 15, 7389–7394. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, M.Y.; Son, H.Y.; Park, B.K.; Ryu, S.Y.; Jung, J.Y. Red ginseng ameliorates acute cisplatin-induced nephropathy. Planta Med. 2014, 80, 645–654. [Google Scholar] [CrossRef]
- Ibrahim, A.; Eldaim, M.A.; Abdel-Daim, M.M. Nephroprotective effect of bee honey and royal jelly against subchronic cisplatin toxicity in rats. Cytotechnology 2016, 68, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Saad, A.A.; Youssef, M.I.; El-Shennawy, L.K. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem. Toxicol. 2009, 47, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Kalra, P.; Gupta, Y.K.; Saini, D.; Kumar, A.; Singh, S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016, 7, 3091–3101. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Rajabian, A.; Farzadnia, M.; Hoseini, A.; Poorlashkari, M.; Taghavi, A.; Dolati, K.; Bazmandegan, G. Protective effect of pomegranate seed oil against cisplatin-induced nephrotoxicity in rat. Ren. Fail. 2015, 37, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, F.; Nematbakhsh, M.; Monajemi, R.; Pezeshki, Z.; Talebi, A.; Zolfaghari, B.; Mansoori, A.; Saberi, S.; Dehghani, A.; Ashrafi, F. Effect of pomegranate flower extract on cisplatin-induced nephrotoxicity in rats. J. Nephropathol. 2014, 3, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-H.; Kim, H.-J.; Oh, G.-S.; Shen, A.; Lee, S.; Choe, S.-K.; Park, R.; So, H.-S. Capsaicin ameliorates cisplatin-induced renal injury through induction of heme oxygenase-1. Mol. Cells 2014, 37, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arjumand, W.; Sultana, S. Glycyrrhizic acid: A phytochemical with a protective role against cisplatin-induced genotoxicity and nephrotoxicity. Life Sci. 2011, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 Ameliorates Cisplatin-Induced Nephrotoxicity in Mice through Inhibition of Inflammation, Oxidative Stress, and Apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef] [Green Version]
- Ueki, M.; Ueno, M.; Morishita, J.; Maekawa, N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. J. Biosci. Bioeng. 2012, 115. [Google Scholar] [CrossRef]
- Horváth, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. β-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Sahu, B.D.; Rentam, K.K.R.; Putcha, U.K.; Kuncha, M.; Vegi, G.M.N.; Ramakrishna, S. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem. Toxicol. 2011, 49, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, S.; Nematbakhsh, M.; Bahadorani, M.; Pezeshki, Z.; Talebi, A.; Ghannadi, A.R.; Ashrafi, F. Effects of Fennel Essential Oil on Cisplatin-induced Nephrotoxicity in Ovariectomized Rats. Toxicol. Int. 2013, 20, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahmar, A.; Dhaouefi, Z.; Khlifi, R.; Sioud, F.; Ghedira, L.C. Pituranthos chloranthus Oil as an Antioxidant-Based Adjuvant Therapy against Cisplatin-Induced Nephrotoxicity. J. Toxicol. 2020, 2020, 7054534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, Z.; Zou, X.; Yang, Y.; Qiu, Y.; Wen, Y. Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int. J. Clin. Exp. Pathol. 2014, 7, 8391–8400. [Google Scholar]
- Qi, Z.L.; Wang, Z.; Li, W.; Hou, J.G.; Liu, Y.; Li, X.D.; Li, H.P.; Wang, Y.P. Nephroprotective Effects of Anthocyanin from the Fruits of Panax ginseng (GFA) on Cisplatin-Induced Acute Kidney Injury in Mice. Phytother. Res. PTR 2017, 31, 1400–1409. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, G.; Hu, Z.; Li, X.; Yang, X.; Jiang, B.; Li, X. Grape seed proanthocyanidin extract protects from cisplatin- induced nephrotoxicity by inhibiting endoplasmic reticulum stress- induced apoptosis. Mol. Med. Rep. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.F.; Yang, C.M.; Su, C.M.; Hu, M.L. Vitamin C protects against cisplatin-induced nephrotoxicity and damage without reducing its effectiveness in C57BL/6 mice xenografted with Lewis lung carcinoma. Nutr. Cancer 2014, 66, 1085–1091. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Naseem, I. Ameliorative effect of riboflavin on the cisplatin induced nephrotoxicity and hepatotoxicity under photoillumination. Food Chem. Toxicol. 2010, 48, 2052–2058. [Google Scholar] [CrossRef]
- Sahu, A.K.; Verma, V.K.; Mutneja, E.; Malik, S.; Nag, T.C.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol. Cell. Biochem. 2019, 452, 141–152. [Google Scholar] [CrossRef]
- Younis, N.S.; Mohamed, M.E. β-Caryophyllene as a Potential Protective Agent Against Myocardial Injury: The Role of Toll-Like Receptors. Molecules 2019, 24, 1929. [Google Scholar] [CrossRef] [Green Version]
- Brooks, C.; Wei, Q.; Cho, S.-G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N.; Mahran, Y.F. Indole-3-carbinol protects against cisplatin-induced acute nephrotoxicity: Role of calcitonin gene-related peptide and insulin-like growth factor-1. Sci. Rep. 2016, 6, 29857. [Google Scholar] [CrossRef]
- Liang, H.; Liu, H.-Z.; Wang, H.-B.; Zhong, J.-Y.; Yang, C.-X.; Zhang, B. Dexmedetomidine protects against cisplatin-induced acute kidney injury in mice through regulating apoptosis and inflammation. Inflamm. Res. 2017, 66, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilmes, A.; Bielow, C.; Ranninger, C.; Bellwon, P.; Aschauer, L.; Limonciel, A.; Chassaigne, H.; Kristl, T.; Aiche, S.; Huber, C.G.; et al. Mechanism of cisplatin proximal tubule toxicity revealed by integrating transcriptomics, proteomics, metabolomics and biokinetics. Toxicol. Vitro 2015, 30, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guáqueta, A.M.; Hobbie, F.; Keerthi, A.; Oun, A.; Kortholt, A.; Boddeke, E.; Dolga, A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019, 118, 109295. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid. Based Complement Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Seol, G.H.; Kang, P.; Lee, H.S.; Seol, G.H. Antioxidant activity of linalool in patients with carpal tunnel syndrome. BMC Neurol. 2016, 16, 17. [Google Scholar] [CrossRef] [Green Version]
- Aleksunes, L.M.; Goedken, M.J.; Rockwell, C.E.; Thomale, J.; Manautou, J.E.; Klaassen, C.D. Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J. Pharm. Exp. Ther. 2010, 335, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Yu, L.; Qiu, J.; Shen, B.; Wang, D.; Soromou, L.W.; Feng, H. Linalool attenuates lung inflammation induced by Pasteurella multocida via activating Nrf-2 signaling pathway. Int. Immunopharmacol. 2014, 21, 456–463. [Google Scholar] [CrossRef]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 2017, 174, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, O.; Zhou, F.; Li, Q.; Wu, Z.; Zheng, Y. Linalool Inhibits LPS-Induced Inflammation in BV2 Microglia Cells by Activating Nrf2. Neurochem. Res. 2015, 40, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Huang, H. Protective effect of linalool against lipopolysaccharide/d-galactosamine-induced liver injury in mice. Int. Immunopharmacol. 2014, 23, 523–529. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Hsu, J.S.; Li, C.C.; Chen, K.M.; Liu, C.T. Protective effect of leaf essential oil from Cinnamomum osmophloeum Kanehira on endotoxin-induced intestinal injury in mice associated with suppressed local expression of molecules in the signaling pathways of TLR4 and NLRP3. PLoS ONE 2015, 10, e0120700. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol. Res. 2016, 65, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Michel, H.E.; Menze, E.T. Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-κB and activating Nrf2 and PPAR-γ signaling pathways. Eur. J. Pharmacol. 2019, 857, 172422. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Gargouri, M.; Dhifi, W.; Ben Saad, R.; Sayahi, N.; Mnif, W.; Saibi, W. Potential anti-inflammatory and antioxidant effects of Citrus aurantium essential oil against carbon tetrachloride-mediated hepatotoxicity: A biochemical, molecular and histopathological changes in adult rats. Environ. Toxicol. 2019, 34, 388–400. [Google Scholar] [CrossRef]
- Iwasaki, K.; Zheng, Y.W.; Murata, S.; Ito, H.; Nakayama, K.; Kurokawa, T.; Sano, N.; Nowatari, T.; Villareal, M.O.; Nagano, Y.N.; et al. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016, 22, 9765–9774. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Ma, J.H.; Fu, Y.; Zhao, H.; Ye, X.X.; Han, Z.; Jia, F.J.; Li, X. Essential oil extracted from erythrina corallodendron L. leaves inhibits the proliferation, migration, and invasion of breast cancer cells. Medicine 2019, 98, e17009. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Cho, Y.J.; Cho, S.K.; Byeon, Y.; Jeon, H.N.; Kim, H.S.; Kim, B.G.; Bae, D.S.; Lopez-Berestein, G.; Sood, A.K.; et al. Linalool-Incorporated Nanoparticles as a Novel Anticancer Agent for Epithelial Ovarian Carcinoma. Mol. Cancer Ther. 2016, 15, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickers, D.; Calow, P.; Greim, H.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H.; Panel, T.R.E. A toxicologic and dermatologic assessment of linalool and related esters when used as fragrance ingredients. Food Chem. Toxicol. 2003, 41, 919–942. [Google Scholar] [CrossRef]








| TLR Pathway Mediators | Primer Sequence (5′ to 3′) | |
|---|---|---|
| Forward Primer | Reverse Primers | |
| TLR4 | AGTGTATCGGTGGTCAGTGTGCT | AAACTCCAGCCACACATTCC |
| MyD88 | GAGATCCGCGAGTTTGAGAC | CTGTTTCTGCTGGTTGCGTA |
| TRIF | TCAGCCATTCTCCGTCCTCTTC | GGTCAGCAGAAGGATAAGGAA |
| Bax | AGACACCTGAGCTGACCTTGGA | CGCTCAGCTTCTTGGTGGAT |
| Bcl2 | GGGATGCCTTTGTGGAACTATATG | CAGCCAGGAGAAATCAAACAGA |
| HMGB-1 | AGGCTGACAAGGCTCGTTATG | TGTCATCCGCAGCAGTGTTG |
| β-Actin | CACGATGGAGGGGCCGGACTCATC | TAAAGACCTCTATGCCAACACAGT |
| Treated Groups | Mortality Rate | Body Weight (gm) | Kidney Index | Blood Urea Nitrogen (BUN) (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|---|---|---|
| Before Treatment | After Treatment | |||||
| Normal | 0/10 | 190 ±8.68 | 220 ±10.75 | 0.71 ± 0.05 | 35.75 ± 3.7 | 0.38 ± 0.12 |
| Linalool | 0/10 | 200 ±11.46 | 240 ±12.47 | 0.74 ± 0.12 | 37.75 ± 2.82 | 0.46 ± 0.85 |
| Cisplatin | 2/10 | 189.42 ± 9.42 | 150.42 ± 7.46 # | 0.94 ± 0.05 # | 174.33 ± 14.50 # | 3.21 ± 0.16 # |
| Cisplatin + linalool (50 mg/kg) | 0/10 | 196.00 ± 14.75 | 185.00 ± 12.76 | 0.57 ± 0.10 * | 70.83 ± 2.40 * | 1. 53 ± 0.05 * |
| Cisplatin + linalool (100 mg/kg) | 0/10 | 184.00 ± 13.5 | 178.00 ± 13.7 | 0.55 ± 0.08 * | 67.83 ± 2.40 * | 1.46 ± 0.05 * |
| Treated Groups | GSH (μmol/g Tissue) | SOD (U/mg Protein) | CAT (nmol/g Tissue) | MDA (nmol/g Tissue) | NADPH (ng/mg Protein) |
|---|---|---|---|---|---|
| Normal | 2.91 ± 0.12 | 16.22 ± 2.84 | 22.70 ± 0.66 | 12.20 ± 0.96 | 1.39 ± 0.35 |
| Linalool | 2.75 ± 0.26 | 19.48 ± 1.99 | 24.03 ± 0.87 | 14.43 ± 0.81 | 1.77 ± 0.67 |
| Cisplatin | 0.56 ± 0.08 # | 3.06 ± 0.69 # | 83.23 ± 5.34 # | 92.83 ± 6.17 # | 9.24 ± 0.88 # |
| Cisplatin +linalool (50 mg/kg) | 1.97 ± 0.31 * | 11.03 ± 1.04 * | 34.40 ± 6.6 * | 34.96 ± 3.04 * | 2.46 ± 0.72 * |
| Cisplatin +linalool (100 mg/kg) | 2.27 ± 0.51 *,@ | 13.87 ± 1.31 *,@ | 28.65 ± 3.73 *,@ | 30.64 ± 2.15 * | 2.01 ± 0.68 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.E.; Abduldaium, Y.S.; Younis, N.S. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways. Biomolecules 2020, 10, 1488. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10111488
Mohamed ME, Abduldaium YS, Younis NS. Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways. Biomolecules. 2020; 10(11):1488. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10111488
Chicago/Turabian StyleMohamed, Maged E., Yamen S. Abduldaium, and Nancy S. Younis. 2020. "Ameliorative Effect of Linalool in Cisplatin-Induced Nephrotoxicity: The Role of HMGB1/TLR4/NF-κB and Nrf2/HO1 Pathways" Biomolecules 10, no. 11: 1488. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10111488