Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities
Abstract
:1. Introduction
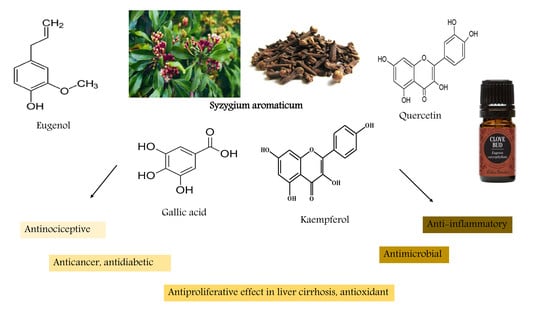
2. Chemical Constituents
3. Crude Clove Extracts Efficacies
4. Biological Activities
4.1. Biological/Biochemical Properties of S. aromaticum
4.2. Efficacy in Diseases
4.3. Efficacy of the Most Common Compound Eugenol
5. Pharmacokinetics Studies of Eugenol
6. Toxicity Doses
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Abushouk, A.I.; Negida, A.; Ahmed, H.; Abdel-Daim, M.M. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity: Future applications in Parkinson’s disease. Biomed. Pharmacother. 2017, 85, 635–645. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharmacother. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Uncaria tomentosa bark, Myrtus communis roots, Origanum vulgare leaves and Cuminum cyminum seeds extracts against the growth of Babesia and Theileria in vitro. Jap. J. Vet. Parasitol. 2018, 17, 1–13. [Google Scholar]
- Beshbishy, A.M.; Batiha, G.E.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on the growth of Babesia and Theileria. Asian Pac. J. Trop. Med. 2019, 12, 425–434. [Google Scholar]
- Cortés-Rojas, D.F.; de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Med. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick. Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Chomchalow, N. Spice production in Asia—An overview. In Proceedings of the Conference IBC’s Asia Spice Markets 96 Conference, Singapore, 27–28 May 1996. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Hu, F.B.; Willett, W.C. Optimal diets for prevention of coronary heart disease. JAMA 2002, 288, 2569–2578. [Google Scholar] [CrossRef]
- Astuti, R.I.; Listyowati, S.; Wahyuni, W.T. Life span extension of model yeast Saccharomyces cerevisiae upon ethanol derived-clover bud extract treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012059. [Google Scholar] [CrossRef]
- Sarrami, N.; Pemberton, M.; Thornhill, M.; Theaker, E.D. Adverse reactions associated with the use of eugenol in dentistry. Br. Dental J. 2002, 193, 253. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.S.; Yadav, A.; Srivastava, S.; Paswan, S.; Dutta, A.S. Recent trends in Indian traditional herbs Syzygium aromaticum and its health benefits. J. Pharmaco. Phytochem. 2012, 1, 13–23. [Google Scholar]
- Martínez-Herrera, A.; Pozos-Guillén, A.; Ruiz-Rodríguez, S.; Garrocho-Rangel, A.; Vértiz-Hernández, A.; Escobar-García, D.M. Effect of 4-Allyl-1-hydroxy-2-methoxybenzene (eugenol) on inflammatory and apoptosis processes in dental pulp fibroblasts. Mediators Inflamm. 2016, 2016, 9371403. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Hisama, M. Suppression of chemical mutagen-induced SOS response by alkylphenols from clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Jirovetz, L.; Buchbauer, G.; Stoilova, I.; Stoyanova, A.; Krastanov, A.; Schmidt, E. Chemical Composition and Antioxidant properties of clove leaf essential oil. J. Agric. Food Chem. 2006, 54, 6303–6307. [Google Scholar] [CrossRef]
- Hastuti, L.T.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Murni, V.W.; Haib, J. The influence of sun drying process and prolonged storage on composition of essential oil from clove buds (Syzygium aromaticum). AIP Confer. Proceed. 2017, 1862, 030092. [Google Scholar]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food. 2011, 14, 975–985. [Google Scholar] [CrossRef]
- Sulaiman, F.A.; Nafiu, M.O.; Yusuf, B.O.; Muritala, H.F.; Adeyemi, S.B.; Omar, S.A.; Dosumu, K.A.; Adeoti, Z.J.; Adegbesan, O.A.; Busari, B.O.; et al. The GC-MS fingerprints of Nicotiana tabacum L. extract and propensity for renal impairment and modulation of serum triglycerides in Wistar rats. J. Pharm. Pharmacogn. Res. 2020, 8, 191–200. [Google Scholar]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of clove (Syzygium aromaticum) oil and its major components to human skin cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef]
- Pawar, V.; Thaker, V. In vitro efficacy of 75 essential oils against Aspergillus niger. Mycoses 2006, 49, 316–323. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzygium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Koba, K.; Nenonene, A.Y.; Raynaud, C.; Chaumont, J.P.; Sanda, K. Antibacterial activities of the buds essential oil of Syzygium aromaticum (L.) Merr. & Perry from Togo. J. Biol. Act. Prod. Nat. 2011, 1, 42–51. [Google Scholar]
- Nassar, M.; Gaara, A.; El-Ghorab, A.; Farrag, A.; Shen, H.; Huq, E.; Mabry, T.J. Chemical constituents of clove (Syzygium aromaticum, Fam. Myrtaceae) and their antioxidant activity. Latinoam. Quim. 2007, 35, 47. [Google Scholar]
- Paarakh, P.M. Terminalia arjuna (Roxb.) Wt. and Arn.: A review. Int. J. Pharmacol. 2010, 6, 515–534. [Google Scholar] [CrossRef]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Joshi, B.; Sah, G.P.; Basnet, B.B.; Bhatt, M.R.; Sharma, D.; Subedi, K.; Pandey, J.; Malla, R. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem). J. Microbiol. Antimicrob. 2011, 3, 1–7. [Google Scholar]
- Oulkheir, S.; Aghrouch, M.; EL Mourabit, F.; Dalha, F.; Graich, H.; Amouch, F.; Ouzaid, K.; Moukale, A.; Chadli, S. Antibacterial activity of essential oils extracts from cinnamon, thyme, clove and geranium against a gram-negative and gram-positive pathogenic bacteria. J. Dis. Med. Plants 2017, 3, 1–5. [Google Scholar]
- Haroun, M.F.; Al-Kayali, R.S. Synergistic effect of Thymbra spicata L. extracts with antibiotics against multidrug-resistant Staphylococcus aureus and Klebsiella pneumoniae strains. Iran. J. Basic Med. Sci. 2016, 19, 1193–1200. [Google Scholar]
- Velluti, A.; Sanchis, V.; Ramos, A.; Turon, C.; Marín, S. Impact of essential oils on growth rate, zearalenone and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. J. Appl. Microbiol. 2004, 96, 716–724. [Google Scholar] [CrossRef]
- Lopez, P.; Sanchez, C.; Batlle, R.; Nerín, C. Solid-and vapor-phase antimicrobial activities of six essential oils: Susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 2005, 53, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, C.; Lima, E.; Oliveira, V.; Pereira, F.O.; Souza, E.L.; Lima, I.O.; Navarro, D.F. Sensitivity of fungi isolated from onychomycosis to Eugenia cariophyllata essential oil and eugenol. Fitoterapia 2005, 76, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Zmantar, T.; Ksouri, R.; Hajlaoui, H.; Mahdouani, K.; Abdelly, C.; Bakhrouf, A. Antioxidant properties of the essential oil of Eugenia caryophyllata and its antifungal activity against a large number of clinical Candida species. Mycoses 2007, 50, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Nejad, S.M.; Özgüneş, H.; Başaran, N. Pharmacological and toxicological properties of eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm. Biol. 2017, 55, 1619–1622. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Rapp, R.P. Changing strategies for the management of invasive fungal infections. Pharmacotherapy 2004, 24, 4S–28S. [Google Scholar] [CrossRef]
- Bouchentouf, S.; Said, G.; Noureddine, M.; Hocine, A.; Angelika, B.A. A note study on antidiabetic effect of main molecules contained in clove using molecular modeling interactions with DPP-4 enzyme. Int. J. Comput. Theor. Chem. 2017, 5, 9–13. [Google Scholar]
- Koh, T.; Murakami, Y.; Tanaka, S.; Machino, M.; Sakagami, H. Re-evaluation of anti-inflammatory potential of eugenol in IL-1beta-stimulated gingival fibroblast and pulp cells. In Vivo 2013, 27, 269–273. [Google Scholar]
- Shukri, R.; Mohamed, S.; Mustapha, N.M. Cloves protect the heart, liver and lens of diabetic rats. Food Chem. 2010, 122, 1116–1121. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.K.; Ahn, Y.J. Acaricidal activity of clove bud oil compounds against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Agric. Food Chem. 2003, 51, 885–889. [Google Scholar] [CrossRef]
- Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 2019, 9, E14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwakeel, H.; Moneim, H.; Farid, M.; Gohar, A.A. Clove oil cream: A new effective treatment for chronic anal fissure. Colorectal Dis. 2007, 9, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasit Vectors. 2019, 12, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. PLoS Negl. Trop. Dis. 2019, 13, e0007030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Latif, A.; Qasmi, I.A. Effect of 50% ethanolic extract of Syzygium aromaticum (L.) Merr. & Perry. (Clove) on the sexual behaviour of normal male rats. BMC Complement. Altern. Med. 2004, 4, 17. [Google Scholar]
- Issac, A.; Gopakumar, G.; Kuttan, R.; Maliakel, B.; Krishnakumar, I.M. Safety and anti-ulcerogenic activity of a novel polyphenol-rich extract of clove buds (Syzygium aromaticum L). Food Funct. 2015, 6, 842–852. [Google Scholar] [CrossRef]
- Johannah, N.; Renny, R.; Gopakumar, G.; Balu, M.; Sureshkumar, D.; Krishnakumar, I.M. Beyond the flavour: A de-flavored polyphenol-rich extract of clove buds (Syzygium aromaticum L) as a novel dietary antioxidant ingredient. Food Funct. 2015, 6, 3373–3382. [Google Scholar]
- Hochenegg, B. Evaluation of the traditional and well-established use of Tormentillae rhizoma, Caryophylli flos and Caryophylli aetheroleum. Thesis Univ. Wien Germany Uniwien 2010. [Google Scholar] [CrossRef]
- Sofia, P.K.; Prasad, R.; Vijay, V.K.; Srivastava, A.K. Evaluation of antibacterial activity of Indian spices against common foodborne pathogens. Int. J. Food Sci. Technol. 2007, 42, 910–915. [Google Scholar] [CrossRef]
- Dorman, H.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Thielmann, J.; Muranyi, P.; Kazman, P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon 2019, 5, e01860. [Google Scholar] [CrossRef] [Green Version]
- Mytle, N.; Anderson, G.; Doyle, M.; Smith, M.A. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control 2006, 17, 102–107. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Micr. 2011, 42, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Gwak, K.S.; Yang, I.; Choi, W.S.; Jo, H.J.; Chang, J.W.; Jeung, E.B.; Choi, I.G. Antifungal activities of the essential oils in Syzygium aromaticum (L.) Merr. Et Perry and Leptospermum petersonii Bailey and their constituents against various dermatophytes. J. Microbiol. 2007, 45, 460–465. [Google Scholar] [PubMed]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of Australian plant extracts against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). J. Basic Microbiol. 2002, 42, 444–448. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Alternat. Med. 2011, 2011, 8. [Google Scholar] [CrossRef] [Green Version]
- Hussein, G.; Miyashiro, H.; Nakamura, N.; Hattori, M.; Kakiuchi, N.; Shimotohno, K. Inhibitory effects of Sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother. Res. 2000, 14, 510–516. [Google Scholar] [CrossRef]
- Minami, M.; Kita, M.; Nakaya, T.; Yamamoto, T.; Kuriyama, H.; Imanishi, J. The inhibitory effect of essential oils on herpes simplex virus type-1 replication in vitro. Microbiol. Immunol. 2003, 47, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Nolkemper, S.; Reichling, J.; Stintzing, F.C.; Carle, R.; Schnitzler, P. Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med. 2006, 72, 1378–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties--an overview. Forsch. Komplementmed. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilling, D.H.; Kitajima, M.; Torrey, J.R.; Bright, K.R. Mechanisms of antiviral action of plant antimicrobials against Murine norovirus. Appl. Environ. Microbiol. 2014, 80, 4898–4910. [Google Scholar] [CrossRef] [Green Version]
- Aboubakr, H.A.; Nauertz, A.; Luong, N.T.; Agrawal, S.; El-Sohaimy, S.A.; Youssef, M.M.; Goyal, S.M. In vitro antiviral activity of clove and ginger aqueous extracts against Feline Calicivirus, a Surrogate for Human Norovirus. J. Food Prot. 2016, 79, 1001–1112. [Google Scholar] [CrossRef]
- Manohar, V.; Ingram, C.; Gray, J.; Talpur, N.A.; Echard, B.W.; Bagchi, D.; Preuss, H.G. Antifungal activities of origanum oil against Candida albicans. Mol. Cell Biochem. 2001, 228, 111–117. [Google Scholar] [CrossRef]
- Tampieri, M.P.; Galuppi, R.; Macchioni, F.; Carelle, M.S.; Falcioni, L.; Cioni, P.L.; Morelli, I. The inhibition of Candida albicans by selected essential oils and their major components. Mycopathologia 2005, 159, 339–345. [Google Scholar] [CrossRef]
- Núñez, L.; D’aquino, M.; Chirife, J. Antifungal properties of clove oil (Eugenia caryophylata) in sugar solution. Braz. J. Microb. 2001, 32, 123–126. [Google Scholar] [CrossRef]
- Chami, F.; Chami, N.; Bennis, S.; Bouchikhi, T.; Remmal, A. Oregano and clove essential oils induce surface alteration of Saccharomyces cerevisiae. Phytother. Res. 2005, 19, 405–408. [Google Scholar] [CrossRef]
- Siripornvisal, S.; Rungprom, W.; Sawatdikarn, S. Antifungal activity of essential oils derived from some medicinal plants against grey mould (Botrytis cinerea). As. J. Food Ag-Ind. 2009, 2, S229–S233. [Google Scholar]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; Costa-de-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagavan, A.; Rahuman, A.A.; Kaushik, N.K.; Sahal, D. In vitro antimalarial activity of medicinal plant extracts against Plasmodium falciparum. Parasitol. Res. 2011, 108, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Kadivar, M.; Karamat, J. Evaluation of phenolic content and antioxidant activity of Iranian caraway in comparison with clove and BHT using model systems and vegetable oil. Int. J. Food Sci. Technol. 2006, 41, 20–27. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil–A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Gülçin, Ì.; Şat, İ.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.İ. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Mehta, K.D.; Garg, G.R.; Mehta, A.K.; Arora, T.; Sharma, A.K.; Khanna, N.; Tripathi, A.K.; Sharma, K.K. Reversal of propoxur-induced impairment of memory and oxidative stress by 4′-chlorodiazepam in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2010, 381, 1. [Google Scholar] [CrossRef]
- Halder, S.; Mehta, A.K.; Kar, R.; Mustafa, M.; Mediratta, P.K.; Sharma, K.K. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011, 77, 830–834. [Google Scholar] [CrossRef]
- Shields, K.M.; McQueen, C.E.; Bryant, P.J. National survey of dietary supplement resources at drug information centers. J. Amer. Pharm. Assoc. 2004, 44, 36–40. [Google Scholar] [CrossRef]
- Vriens, J.; Nilius, B.; Vennekens, R. Herbal compounds and toxins modulating TRP channels. Curr. Neuropharmacol. 2008, 6, 79–96. [Google Scholar]
- Daniel, A.N.; Sartoretto, S.M.; Schmidt, G.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Anti-inflammatory and antinociceptive activities A of eugenol essential oil in experimental animal models. Revista Brasileira de Farmacognosia 2009, 19, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Ogunwande, I.; Olawore, N.; Ekundayo, O.; Walker, T.M.; Schmidt, J.M.; Setzer, W.N. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Yoo, C.B.; Han, K.T.; Cho, K.S.; Ha, J.; Park, H.J.; Nam, J.H.; Kil, U.H.; Lee, K.T. Eugenol isolated from the essential oil of Eugenia caryophyllata induces reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 2005, 225, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Wu, S.J.; Lin, C.C. The anticancer properties and apoptosis-inducing mechanisms of cinnamaldehyde and the herbal prescription Huang-Lian-Jie-Du-Tang (Huáng Lián Jiě Dú Tang) in human hepatoma cells. J. Tradit. Complement. Med. 2013, 3, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Zmantar, T.; Bakhrouf, A. Anticariogenic and cytotoxic activity of clove essential oil (Eugenia caryophyllata) against a large number of oral pathogens. Ann. Microb. 2010, 60, 599–604. [Google Scholar] [CrossRef]
- Kumar, P.; Febriyanti, R.; Sofyan, F.; Luftimas, D.E.; Abdulah, R. Anticancer potential of Syzygium aromaticum L. in MCF-7 human breast cancer cell lines. Pharmac. Res. 2014, 6, 350–354. [Google Scholar] [CrossRef] [Green Version]
- El-Hadary, A.E.; Ramadan Hassanien, M.F. Hepatoprotective effect of cold-pressed Syzygium aromaticum oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. Pharmaceut. Biol. 2016, 54, 1364–1372. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Prasad, R.; Mahmood, A.; Routray, I.; Shinkafi, T.S.; Sahin, K.; Kucuk, O. Eugenol-rich fraction of Syzygium aromaticum (Clove) reverses biochemical and histopathological changes in liver cirrhosis and inhibits hepatic cell proliferation. J. Cancer Prevent. 2014, 19, 288–300. [Google Scholar] [CrossRef]
- Shyamala, M.P.; Venukumar, M.R.; Latha, M.S. Antioxidant potential of the Syzygium aromaticum (gaertn) linn (cloves) in rats fed with high-fat diet. Indian J. Pharmacol. 2003, 35, 99–103. [Google Scholar]
- Kuroda, M.; Mimaki, Y.; Ohtomo, T.; Yamada, J.; Nishiyama, T.; Mae, T.; Kishida, H.; Kawada, T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J. Nat. Med. 2012, 66, 394–399. [Google Scholar] [CrossRef]
- Sanae, F.; Kamiyama, O.; Ikeda-Obatake, K.; Higashi, Y.; Asano, N.; Adachi, I.; Kato, A. Effects of eugenol-reduced clove extract on glycogen phosphorylase b and the development of diabetes in db/db mice. Food Funct. 2014, 5, 214–219. [Google Scholar] [CrossRef]
- Okasha, X.; Magaji, R.; Abubakar, M.S.; Fatihu, M.Y. Effects of ethyl acetate portion of Syzygium aromaticum flower bud extract on Indomethacin-induced gastric ulceration and gastric secretion. Eur. J. Sci. Res. 2008, 20, 905–913. [Google Scholar]
- Przygodzka, M.; Zieliński, H.; Ciesarová, Z.; Kukurová, K.; Lamparski, G. Effect of selected spices on chemical and sensory markers in fortified rye-buckwheat cakes. Food Sci. Nutr. 2016, 4, 651–660. [Google Scholar] [CrossRef]
- Jung, C.H.; Ahn, J.; Jeon, T.I.; Kim, T.W.; Ha, T.Y. Syzygium aromaticum ethanol extract reduces high-fat diet-induced obesity in mice through downregulation of adipogenic and lipogenic gene expression. Exp. Ther. Med. 2012, 4, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Pulikottil, S.J.; Nath, S. Potential of clove of Syzygium aromaticum in development of a therapeutic agent for periodontal disease: A review. S. Afr. Dent. J. 2015, 70, 108–115. [Google Scholar]
- Tajuddin, A.S.; Latif, A.; Qasmi, I.A. Aphrodisiac activity of 50% ethanolic extracts of Myristica fragrans Houtt. (Nutmeg) and Syzygium aromaticum (L) Merr. & Perry. (Clove) in male mice: A comparative study. BMC Complement. Altern. Med. 2003, 3, 6. [Google Scholar]
- Saeed, S.A.; Simjee, R.U.; Shamim, G.; Gilani, A.H. Eugenol: A dual inhibitor of platelet-activating factor and arachidonic acid metabolism. Phytomedicine 1995, 2, 23–28. [Google Scholar] [CrossRef]
- Raghavendra, R.H.; Naidu, K.A. Spice active principles as the inhibitors of human platelet aggregation and thromboxane biosynthesis. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 73–78. [Google Scholar] [CrossRef]
- Lima, F.C.; Peixoto-Neves, D.; Gomes, M.D.; Coelho-de-Souza, A.N.; Lima, C.C.; Araújo Zin, W.; Magalhães, P.J.; Saad, L.; Leal-Cardoso, J.H. Antispasmodic effects of eugenol on rat airway smooth muscle. Fundam. Clin. Pharmacol. 2011, 25, 690–699. [Google Scholar] [CrossRef]
- Guénette, S.A.; Ross, A.; Marier, J.F.; Beaudry, F.; Vachon, P. Pharmacokinetics of eugenol and its effects on thermal hypersensitivity in rats. Eur. J. Pharmacol. 2007, 562, 60–67. [Google Scholar] [CrossRef]
- Tao, G.; Irie, Y.; Li, D.J.; Keung, W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005, 13, 4777–4788. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, T.F.; de Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. Clove and eugenol in noncytotoxic concentrations exert immunomodulatory/anti-inflammatory action on cytokine production by murine macrophages. J. Pharm. Pharmacol. 2012, 64, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.G.; Fernandes, A., Jr.; Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Nat. Prod. Res. 2009, 23, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Ueda-Nakamura, T.; Mendonça-Filho, R.R.; Morgado-Díaz, J.A.; Korehisa Maza, P.; Prado Dias Filho, B.; Aparício Garcia Cortez, D.; Alviano, D.S.; Rosa Mdo, S.; Lopes, A.H.; Alviano, C.S.; et al. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006, 55, 99–105. [Google Scholar] [CrossRef]
- Santoro, G.F.; Cardoso, M.G.; Guimarães, L.G.; Mendonça, L.Z.; Soares, M.J. Trypanosoma cruzi: activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007, 116, 283–290. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.; Cavaleiro, C.; Sousa, M.C. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ahmad, A.A.; Hassan, T.M.; El-Deek, H.E.M.; Fouad, S.S.; Althagfan, S.S. Eugenol, a potential schistosomicidal agent with anti-inflammatory and antifibrotic effects against Schistosoma mansoni, induced liver pathology. Infect. Drug Resist. 2019, 12, 709–719. [Google Scholar] [CrossRef] [Green Version]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—from the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Leem, H.H.; Kim, E.O.; Seo, M.J.; Choi, S.W. Antioxidant and anti-inflammatory activities of eugenol and its derivatives from clove (Eugenia caryophyllata Thunb.). J. Korean Soc. Food Sci. Nutr. 2011, 40, 1361–1370. [Google Scholar] [CrossRef]
- Grespan, R.; Paludo, M.; de Paula Lemos, H.; Barbosa, C.P.; Bersani-Amado, C.A.; Dalalio, M.M.; Cuman, R.K. Anti-arthritic effect of eugenol on collagen-induced arthritis experimental model. Biolog. Pharm. Bull. 2012, 35, 1818–1820. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.S.; Oh, O.J.; Min, H.Y.; Park, E.J.; Kim, Y.; Park, H.J.; Nam Han, Y.; Lee, S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264. 7 cells. Life Sci. 2003, 73, 337–348. [Google Scholar] [CrossRef]
- Pisano, M.; Pagnan, G.; Loi, M.; Mura, M.E.; Tilocca, M.G.; Palmieri, G.; Fabbri, D.; Dettori, M.A.; Delogu, G.; Ponzoni, M.; et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol. Cancer. 2007, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja, M.R.C.; Srinivasan, V.; Selvaraj, S.; Mahapatra, S.K. Versatile and synergistic potential of eugenol: A review. Pharm. Anal. Acta 2015, 6, 367. [Google Scholar]
- Nam, H.; Kim, M.M. Eugenol with antioxidant activity inhibits MMP-9 related to metastasis in human fibrosarcoma cells. Food Chem. Toxicol. 2013, 55, 106–112. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Combination of phenylpropanoids with 5-fluorouracil as anti-cancer agents against human cervical cancer (HeLa) cell line. Phytomedicine 2013, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Edris, A.E. Protective effect of clove oil and eugenol microemulsions on fatty liver and dyslipidemia as components of metabolic syndrome. J. Med. Food 2014, 17, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Prasad, R.; Naime, M.; Zafar, H.; Mahmood, A.; Routray, I.; Yalniz, M.; Bahcecioglu, İ.H.; Sahin, K. Dried peel fraction of Citrus sinensis partially reverses pathological changes in rat model of liver cirrhosis. Mediterr. J. Nutr. Metabol. 2011, 4, 57–67. [Google Scholar] [CrossRef]
- Said, M.M. The protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fund. Clin. Pharmac. 2011, 25, 708–716. [Google Scholar] [CrossRef]
- Oliveira, F.; Andrade, L.N.; de Sousa, E.B.; de Sousa, D.P. Anti-ulcer activity of essential oil constituents. Molecules 2014, 19, 5717–5747. [Google Scholar] [CrossRef] [Green Version]
- Pramod, K.; Ansari, S.H.; Ali, J. Eugenol: A natural compound with versatile pharmacological actions. Nat. Prod. Commun. 2010, 5, 1999–2006. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.L.; Cohen, S.M.; Fukushima, S.; Gooderham, N.J.; Hecht, S.S.; Guengerich, F.P.; Rietjens, I.M.C.M.; Bastaki, M.; Harman, C.L.; McGowen, M.M.; et al. The safety evaluation of food flavouring substances: The role of metabolic studies. Toxicol. Res. (Camb) 2018, 7, 618–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayasteltar, L.; Nair, G.G.; Maliakel, B.; Kuttan, R.; Krishnakumar, I.M. Safety assessment of a standardized polyphenolic extract of clove buds: Subchronic toxicity and mutagenicity studies. Toxicol. Rep. 2016, 3, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuj, G.; Sanjay, S. Eugenol: A potential phytochemical with multifaceted therapeutic activities. Pharmacologyonline 2010, 2, 108–120. [Google Scholar]
- Mishra, R.K.; Singh, S.K. Safety assessment of Syzygium aromaticum flower bud (clove) extract with respect to testicular function in mice. Food Chem. Toxic. 2008, 46, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Doleželová, P.; Mácová, S.; Plhalova, L.; Pistekova, V.; Svobodova, Z. The acute toxicity of clove oil to fish Danio rerio and Poecilia reticulata. Acta Vet. Brno 2011, 80, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Janes, S.E.J.; Price, C.S.G.; Thomas, D. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur. J. Ped. 2005, 164, 520–522. [Google Scholar] [CrossRef]

| Compd. | IUPAC Name | Chemical Formula | Compd. | IUPAC Name | Chemical Formula |
|---|---|---|---|---|---|
| Eugenol | 2-Methoxy-4-(prop-2-en-1-yl)phenol | C10H12O2 | Gallic acid | 3,4,5-Trihydroxybenzoic acid | C7H6O5 |
| β-Caryophyllene | (1R,4E,9S)-4,11,11-Trimethyl-8-methylidenebicyclo[7.2.0]undec-4-ene | C15H24 | Biflorin | 5,7-dihydroxy-2-methyl-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]chromen-4-one | C16H18O9 |
| Vanillin | 4-Hydroxy-3-methoxybenzaldehyde | C8H8O3 | Myricetin | 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-4-chromenone | C15H10O8 |
| Crategolic acid (Maslinic acid) | (4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-10,11-dihydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | C30H48O4 | Campesterol | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5,6-dimethylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | C28H48O |
| Kaempferol | 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one | C15H10O6 | Stigmasterol | 3S,8S,9S,10R,13R,14S,17R)-17-[(E,2R,5S)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | C29H48O |
| Rhamnetin | 2-(3,4-dihydroxyphenyl)-3,5-dihydroxy-7-methoxychromen-4-one | C16H12O7 | Oleanolic acid | (4aS,6aR,6aS,6bR,8aR,10S,12aR,14bS)-10-hydroxy-2,2,6a,6b,9,9,12a-heptamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | C30H48O3 |
| Eugenitin | 5-Hydroxy-7-methoxy-2,6-dimethylchromen-4-one | C12H12O4 | Bicornin | [(12R,14S,15R,16R,17R)-4,5,6,22,23,29,30-heptahydroxy-9,19,26-trioxo-14,15-bis[(3,4,5-trihydroxybenzoyl)oxy]-2,10,13,18,25-pentaoxahexacyclo[18.9.3.03,8.012,17.024,32.027,31]dotriaconta-1(29),3,5,7,20,22,24(32),27,30-nonaen-16-yl] 3,4,5-trihydroxybenzoate | C48H32O30 |
| Eugenin | 5-Hydroxy-7-methoxy-2-methylchromen-4-one | C11H10O4 | Quercetin | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | C15H10O7 |
| Ellagic acid | 2,3,7,8-Tetrahydroxy-chromeno[5,4,3-cde]chromene-5,10-dione | C14H6O8 | Carvacrol | 2-methyl-5-propan-2-ylphenol | C10H14O |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10020202
El-Saber Batiha G, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules. 2020; 10(2):202. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10020202
Chicago/Turabian StyleEl-Saber Batiha, Gaber, Luay M. Alkazmi, Lamiaa G. Wasef, Amany Magdy Beshbishy, Eman H. Nadwa, and Eman K. Rashwan. 2020. "Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities" Biomolecules 10, no. 2: 202. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10020202