Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae)
Abstract
:1. Introduction
2. Physicochemical Features
2.1. Chemical Constituents
2.2. Mechanisms of Action
3. Pharmacological Actions
3.1. Traditional Uses of G. glabra
3.2. In Vitro Pharmacological/Biological Properties of G. glabra Extract and Its Metabolites
3.3. In Vivo Pharmacological/Biological Properties of G. glabra Extract and Its Metabolites
3.4. Clinical Efficacy of G. glabra Extract and Its Metabolites
3.5. Pharmacokinetics of G. glabra Extract and Its Metabolites
3.5.1. In Animals
3.5.2. In Humans
3.6. Dose, Side Effects, and Contraindications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HPLC | high-performance liquid chromatography |
| G. glabra | Glycyrrhiza glabra |
| IUPAC | International Union of Pure and Applied Chemistry |
| 11 beta-HSD2 | 11 beta-hydroxysteroid dehydrogenase |
| HIV | human immunodeficiency virus |
| ROS | reactive oxygen species |
| MAO | monoamine oxidase |
| GAMG | glycyrrhetinic acid monoglucuronide acetylated |
| SARS | severe acute respiratory syndrome |
| DGL | deglycyrrhizinated licorice |
| STZ | streptozotocin |
| PAF | platelet aggregating factor |
| CCh | carbamylcholine |
| L-NAME | N-w-nitro-L-arginine methyl ester |
| AD | Alzheimer’s Disease |
| SCA3 | spinocerebellar ataxia type 3 |
| NFE2L2-ARE | nuclear factor erythroid 2-related factor 2-antioxidant-responsive elements |
| PPARGC1A | coactivator 1α |
| AUC | areas under the plasma-time curve |
| LD50 | half-maximal lethal concentrations. |
References
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. Evaluation of the inhibitory effect of ivermectin on the growth of Babesia and Theileria parasites in vitro and in vivo. Trop. Med. Health. 2019, 47, 42. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Uncaria tomentosa bark, Myrtus communis roots, Origanum vulgare leaves and Cuminum cyminum seeds extracts against the growth of Babesia and Theileria in vitro. Jap. J. Vet. Parasitol. 2018, 17, 1–13. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.A.; Adeyemi, O.S.; Nadwa, E.; Rashwan, E.; Yokoyama, N.; Igarashi, I. Safety and efficacy of hydroxyurea and eflornithine against most blood parasites Babesia and Theileria. PLoS ONE 2020, 15, e0228996. [Google Scholar]
- Batiha, G.-S.; Beshbishy, A.M.; Alkazmi, L.M.; Adeyemi, O.S.; Nadwa, E.H.; Rashwan, E.K.; El-Mleeh, A.; Igarashi, I. Gas chromatography-mass spectrometry analysis, phytochemical screening and antiprotozoal effects of the methanolic Viola tricolor and acetonic Laurus nobilis extracts. BMC Complement. Altern. Med. 2020, in press. [Google Scholar]
- Beshbishy, A.M.; Batiha, G.E.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Inhibitory effects of methanolic Olea europaea and acetonic Acacia laeta on the growth of Babesia and Theileria. Asian Pac. J. Trop. Med. 2019, 12, 425–434. [Google Scholar]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Shaheen, M.H.; Yokoyama, N.; Igarashi, I. Inhibitory effects of Syzygium aromaticum and Camellia sinensis methanolic extracts on the growth of Babesia and Theileria parasites. Ticks Tick. Borne Dis. 2019, 10, 949–958. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.A.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protoz. Res. 2019, 29, 8–25. [Google Scholar]
- Batiha, G.-S.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Beshbishy, A.M.; Batiha, G.E.-S.; Alkazmi, L.; Nadwa, E.; Rashwan, E.; Abdeen, A.; Yokoyama, N.; Igarashi, I. Therapeutic Effects of Atranorin towards the Proliferation of Babesia and Theileria Parasites. Pathogen 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Beshbishy, A.M.; Batiha, G.E.; Yokoyama, N.; Igarashi, I. Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo. Parasit Vectors. 2019, 12, 269. [Google Scholar] [CrossRef] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory effects of Glycyrrhiza glabra and its major constituent Glycyrrhizin on inflammation-associated corneal neovascularization. Evid. Based Complement. Alternate. Med. 2018, 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian medicinal plants. New Delhi NISCAIR CSIR 2002, 1956–1992. [Google Scholar]
- Kriker, S.; Yahia, A.; Nebbache, S. Effect of climate on some morphological and chemical characteristics of the plant Glycyrrhiza glabra L. in two arid regions of southern Algeria. Egypt. Acad. J. Biol. Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Sawant, B.S.; Alawe, J.R.; Rasal, K.V. Pharmacognostic study of Glycyrrhiza glabra Linn- a review. Inter. Ayurv. Med. J. 2016. [Google Scholar]
- Husain, A.; Ahmad, A.; Mujeeb, M.; Khan, S.A.; Alghamdi, A.G.; Anwar, F. Quantitative analysis of total phenolic, flavonoid contents and HPTLC fingerprinting for standardization of Glycyrrhiza glabra Linn. roots. Herb. Med. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Biondi, D.M.; Rocco, C.; Ruberto, G. New dihydrostilbene derivatives from the leaves of Glycycrrhiza glabra and evaluation of their antioxidant activity. J. Nat. Prod. 2003, 66, 477–480. [Google Scholar] [CrossRef]
- Washington, D.C. Food Chemicals Codex, 5th ed.; National Academy Press: Washington, DC, USA, 2003; Volume 25. [Google Scholar]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 4, 167–192. [Google Scholar] [CrossRef]
- Badr, S.E.A.; Sakr, D.M.; Mahfouz, S.A.; Abdelfattah, M.S. Licorice (Glycyrrhiza glabra L.): Chemical composition and biological impacts. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 606–621. [Google Scholar]
- Sabbadin, C.; Bordin, L.; Donà, G.; Manso, J.; Avruscio, G.; Armanini, D. Licorice: From pseudohyperaldosteronism to therapeutic uses. Front. Endocrinol. (Lausanne) 2019, 10, 484. [Google Scholar] [CrossRef] [Green Version]
- Calò, L.A.; Zaghetto, F.; Pagnin, E.; Davis, P.A.; De Mozzi, P.; Sartorato, P.; Martire, G.; Fiore, C.; Armanini, D. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22(phox) in human mononuclear leukocytes. J. Clin. Endocrinol. Metab. 2004, 89, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Omar, H.R.; Komarova, I.; El-Ghonemi, M.; Fathy, A.; Rashad, R.; Abdelmalak, H.D.; Yerramadha, M.R.; Ali, Y.; Helal, E.; Camporesi, E.M. Licorice abuse: Time to send a warning message. Ther. Adv. Endocrinol. Metab. 2012, 3, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kwas, C.; Wu, L. Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission. J. Virol. 2009, 83, 4195–4204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B. 2015, 5, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Harwansh, R.K.; Patra, K.C.; Pareta, S.K.; Singh, J.; Biswas, R. Pharmacological studies on Glycyrrhiza glabra: A review. Pharmacology 2011, 2, 1032–1038. [Google Scholar]
- Lakshmi, T.; Geetha, R.V. Glycyrrhiza glabra Linn. commonly known as licorice: A therapeutic review. Int. J. Pharm. Pharm. Sci. 2011, 3, 20–25. [Google Scholar]
- Khare, C.P. Encyclopaedia of Indian Medicinal Plants; Springer: New York, NY, USA, 2004; pp. 233–235. [Google Scholar]
- Ashok, S. The Herbs of India, 1st ed.; Hi Scan Pvt. Ltd.: Gujrat, India, 2005; Volume 2, p. 566. [Google Scholar]
- Damle, M. Glycyrrhiza glabra (Liquorice)—A potent medicinal herb. Inter. J. Herb. Med. 2014, 2, 132–136. [Google Scholar]
- Kaur, R.; Dhinds, A.S. Glycyrrhiza glabra: A phytopharmacological review. IJPSR 2013, 4, 2470–2477. [Google Scholar]
- Tewari, D.; Mocan, A.; Parvanov, E.D.; Sah, A.N.; Nabavi, S.M.; Huminiecki, L.; Ma, Z.F.; Lee, Y.Y.; Horbańczuk, J.O.; Atanasov, A.G. Ethnopharmacological approaches for therapy of jaundice: Part II. Highly used plant species from Acanthaceae, Euphorbiaceae, Asteraceae, Combretaceae, and Fabaceae families. Front. Pharm. 2017, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, J.B.; Kor, Z.M.; Goftar, M.K. Licorice (Glycyrrhiza glabra Linn) as a valuable medicinal plant. Inter. J. Advance. Biol. Biomed. Res. 2013, 1, 1281–1288. [Google Scholar]
- Jahan, Y.; Siddique, H.H. Study of antitussive potential of Glycyrrhiza glabra & Adhatoda vasica using a cough model induced by SO2 gas in mice. Inter. J. Pharm. Sci. Res. 2012, 3, 1668–1674. [Google Scholar]
- Kuang, Y.; Li, B.; Fan, J.; Qiao, X.; Ye, M. Antitussive and expectorant activities of licorice and its major compounds. Biol. Org. Med. Chem. 2018, 26, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Haque, A.; Hamid, K.; Urmi, K.F.; Roy, S. Antimicrobial, cytotoxic and antioxidant activity of methanolic extract of Glycyrrhiza glabra. Agric. Biol. J. N. Am. 2010, 1, 957–960. [Google Scholar] [CrossRef]
- Rodino, S.; Butu, A.; Butu, M.; Cornea, P.C. Comparative studies on antibacterial activity of licorice, elderberry and dandelion. Digest. J. Nanomat. Biostru. 2015, 10, 947–955. [Google Scholar]
- Kriker, S.; Yahia, A. Effect of flavonoid extract of the medicinal plant (Glycyrrhiza glabra L.) in the region of Djamaa (south of Algeria) on the growth of some human pathogenic bacteria. J. Pharmacogn. PhytoChem. 2013, 2, 58–62. [Google Scholar]
- Nirmala, P.; Selvaraj, T. Anti-inflammatory and anti-bacterial activities of Glycyrrhiza glabra L. J. Agr. Technol. 2011, 7, 815–823. [Google Scholar]
- Sedighinia, F.; Afshar, A.S.; Soleimanpour, S.; Zarif, R.; Asili, J.; Ghazvini, K. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: An in vitro study. Avicenna J. Phytomed. 2012, 2, 118–124. [Google Scholar]
- Irani, M.; Sarmadi, M.; Bernard, F.; Ebrahimipour, G.H.; Bazarnov, H.S. Leaves antimicrobial activity of Glycyrrhiza glabra L. Iran. J. Pharm. Res. 2010, 9, 425–428. [Google Scholar]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumarb, J.K.; Rahuja, N.; Luqmana, S. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef]
- Krausse, R.; Bielenberg, J.; Blaschek, W.; Ullmann, U. In vitro anti-Helicobacter pylori activity of extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. [Google Scholar] [CrossRef] [Green Version]
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046. [Google Scholar] [CrossRef] [Green Version]
- De Simone, F.; Aquino, R.; De Tommasi, N.; Mahmood, N.; Piacente, S.; Pizza, C. Anti-HIV aromatic compounds from higher plants. In Bioactive Compounds from Natural Sources: Isolation, Characterization and Biological Properties; Tringali, C., Ed.; Taylor and Francis: New York, NY, USA, 2001; p. 325. [Google Scholar]
- De Clercq, E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med. Res. Rev. 2000, 20, 323–349. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Miyadera, H.; Kobayashi, T.; Takamiya, S.; Waki, S.; Iwata, S.; Shibata, S.; Kita, K. Parasite mitochondria as a target of chemotherapy: Inhibitory effect of licochalcone A on the Plasmodium falciparum respiratory chain. Ann. N. Y. Acad. Sci. 2005, 1056, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Ming, C.; Andersen, L.; Hjørne, U.; Olsen, C.E.; Cornett, C.; Theander, T.G.; Kharazmi, A. An antileishmanial chalcone from Chinese licorice roots. Planta Med. 1994, 60, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.S.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Shaheen, H.; Yokoyama, N.; Igarashi, I. The effects of trans-chalcone and chalcone 4 hydrate on the growth of Babesia and Theileria. PLoS Negl. Trop. Dis. 2019, 13, e0007030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoomeh, M.J.; Kiarash, G. In vitro susceptibility of Helicobacter pylori to licorice extract. Iran. J. Pharm. Res. 2007, 6, 69–72. [Google Scholar]
- Karahan, F.; Avsar, C.; Ozyigit, I.I.; Berber, I. Antimicrobial and antioxidant activities of medicinal plant Glycyrrhiza glabra var. glandulifera from different habitats. Biotech. Biotech. Equip. 2016, 30, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Agrawal, R.C.; Pandey, S. Phytochemical screening and determination of anti-bacterial and anti-oxidant potential of Glycyrrhiza glabra root extracts. J. Environ. Res. Dev. 2013, 7, 1552–1558. [Google Scholar]
- Adel, M.; Alousi, L.A.; Salem, H.A. Licorice: A possible anti-inflammatory and anti-ulcer drug. AAPS Pharm. Sci. Technol. 2005, 6, 74–82. [Google Scholar]
- Yang, E.J.; Min, J.S.; Ku, H.Y.; Choi, H.S.; Park, M.; Kim, M.; Song, K.S.; Lee, D.S. Isoliquiritigenin isolated from Glycyrrhiza uralensis protects neuronal cells against glutamate-induced mitochondrial dysfunction. Biochem. Biophys. Res. Commun. 2012, 421, 658–664. [Google Scholar] [CrossRef]
- Mendes-Silva, W.; Assafim, M.; Ruta, B.; Monteiro, R.Q.; Guimaraes, J.A.; Zingali, R.B. Antithrombotic effect of glycyrrhizin, a plant-derived thrombin inhibitor. Thromb. Res. 2003, 112, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.Y.H.; Islam, R.; Bhuiyan, S.M.; Islam, A.B.M.R. Standardization, quality control and pharmacological review on Glycyrrhiza glabra L. A potential medicinal herb in Unani and Ayurvedic systems of medicine. Hamdard Med. 2015, 58, 45–72. [Google Scholar]
- Wagner, H.; Jurcic, K. Immunological studies of Revitonil: A phyto pharmaceutical containing Echinacea purpurea and Glycyrrhiza glabra root extract. Phytomedicine 2002, 9, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Blatina, L.A. Chemical modification of glycyrrhizic acid as a route to bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar] [CrossRef]
- Arora, R.; Chawla, R.; Marwah, R.; Arora, P.; Sharma, R.K.; Kaushik, V.; Goel, R.; Kaur, A.; Silambarasan, M.; Tripathi, R.P.; et al. Potential of complementary and alternative medicine in preventive management of novel H1N1 Flu (Swine Flu) pandemic: Thwarting potential disasters in the bud. Evid. Based Complement Alternat. Med. 2011, 2011, 1–16. [Google Scholar] [CrossRef]
- Sheela, M.L.; Ramakrishna, M.K.; Salimath, B.P. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int. Immunopharmacol. 2006, 6, 494–498. [Google Scholar] [CrossRef]
- Salvi, M.; Fiore, C.; Armanini, D.; Toninello, A. Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria. Biochem. Pharmacol. 2003, 66, 2375–2379. [Google Scholar] [CrossRef]
- Fiore, C.; Salvi, M.; Palermo, M.; Sinigagliab, G.; Armaninia, D.; Toninello, A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta 2004, 1658, 195–201. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rashid, M.A. Antimicrobial activity and cytotoxicity of Eclipta prostrata. Orient. Pharm. Exp. Med. 2008, 8, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Agrawal, R.C.; Shrivastava, V.K. Assessment of median lethal dose and antimutagenic effects of Glycyrrhiza glabra root extract against chemically induced micronucleus formation in swiss albino mice. Int. J. Basic. Clin. Pharmacol. 2014, 3, 292–297. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Agrawal, R.C. Evaluation of Anticlastogenic effects of Glycyrrhiza glabra root extract against cyclophosphamide induced chromosomal aberration in swiss albino mice. J. Appl. Pharm. Sci. 2015, 5, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.; Jung, Y.D.; Cheon, S.H. Cytotoxic allyl retrochalcone from the roots of Glycyrrhiza inflate. Chem. Pharm. Bull. 2005, 53, 694–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib Naseri, M.; Arabiyan, M.; Gharib Naseri, Z. Antispasmodic effect of hydroalcoholic leaf extract of licorice ileum contraction in rat. Shahrekord J. Med. Sci. 2008, 9, 1–9. [Google Scholar]
- Ghayedi, N.; Khoshnam, S.E.; Bahaoddini, A. The effect of hydro-alcoholic extract of licorice (Glycyrrhiza glabra) rhizome on the mechanical activity of the colon of male rats and its interaction with adrenergic system. Armaghane Danesh. 2016, 21, 225–237. [Google Scholar]
- Sato, Y.; He, J.X.; Nagai, H.; Tani, T.; Akao, T. Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine. Biol. Pharm. Bull. 2007, 30, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zhu, L.; Liu, Y.; Zhou, Q.; Chen, H.; Yang, J. Isoliquiritigenin, a flavonoid from Licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. Phytother. Res. 2009, 23, 498–506. [Google Scholar] [CrossRef]
- Khoshnazar, S.M.; Bahaoddini, A.; Najafipour, H. Effect of alcoholic extract of licorice (Glycyrrhiza glabra L.) rhizome on isolated duodenum motility in male rats and its interference with cholinergic, nitrergic, and adrenergic systems. Bull. Env. Pharmacol. Life Sci. 2013, 2, 173–177. [Google Scholar]
- Hajirahimkhan, A.; Simmler, C.; Yuan, Y.; Anderson, J.R.; Chen, S.N.; Nikolić, D.; Dietz, B.M.; Pauli, G.F.; van Breemen, R.B.; Bolton, J.L. Evaluation of estrogenic activity of licorice species in comparison with hops used in botanicals for menopausal symptoms. PLoS ONE 2013, 8, e67947. [Google Scholar] [CrossRef]
- Palmer, A.M. Pharmacotherapy for Alzheimer’s disease: Progress and prospects. Trends Pharmacol. Sci. 2002, 23, 426–433. [Google Scholar] [CrossRef]
- Jewart, R.D.; Green, J.; Lu, C.J.; Cellar, J.; Tune, L.E. Cognitive, behavioural, and physiological changes in Alzheimer’s disease patients as a function of incontinence medications. Am. J. Geriatr. Psychiatry 2005, 13, 324–328. [Google Scholar] [CrossRef]
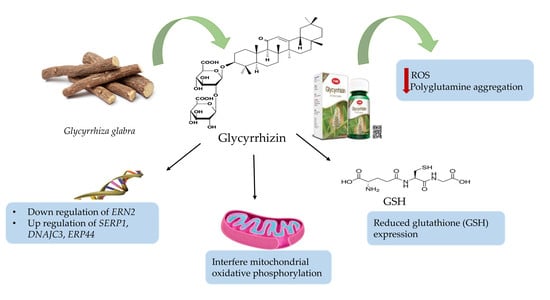
- Chang, K.H.; Chen, I.C.; Lin, H.Y.; Chen, H.C.; Lin, C.H.; Lin, T.H.; Weng, Y.T.; Chao, C.Y.; Wu, Y.R.; Lin, J.Y.; et al. The aqueous extract of Glycyrrhiza inflata can upregulate unfolded protein response-mediated chaperones to reduce tau misfolding in cell models of Alzheimer’s disease. Drug Des. Dev. Ther. 2016, 10, 885–896. [Google Scholar]
- Chen, C.M.; Weng, Y.T.; Chen, W.L.; Lin, T.H.; Chao, C.Y.; Lin, C.H.; Chen, I.C.; Lee, L.C.; Lin, H.Y.; Wu, Y.R.; et al. Aqueous extract of Glycyrrhiza inflata inhibits aggregation by upregulating PPARGC1A and NFE2L2-ARE pathways in cell models of spinocerebellar ataxia 3. Free Radic. Biol. Med. 2014, 71, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative stress in the progression of Alzheimer’s disease in the frontal cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/antioxidant imbalance in Alzheimer’s disease: Therapeutic and diagnostic prospects. Oxid. Med. Cell. Longev. 2018, 2018, 6435861. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Yuan, B.C.; Ma, Y.S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017, 55, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross-talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar]
- Dhingra, D.; Parle, M.; Kulkarni, S.K. Memory enhancing activity of Glycyrrhiza glabra in mice. J. Ethnopharmacol. 2004, 91, 361–365. [Google Scholar] [CrossRef]
- Ikarashi, Y.; Mizoguchi, K. Neuropharmacological efficacy of the traditional Japanese Kampo medicine yokukansan and its active ingredients. Pharmacol. Ther. 2016, 166, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Danysz, W.; Parsons, C.G. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: Preclinical evidence. Int. J. Geriatr. Psychiatry 2003, 18, S23–S32. [Google Scholar] [CrossRef]
- Kanno, H.; Kawakami, Z.; Tabuchi, M.; Mizoguchi, K.; Ikarashi, Y.; Kase, Y. Protective effects of glycycoumarin and procyanidin B1, active components of traditional Japanese medicine yokukansan, on amyloid-beta oligomer-induced neuronal death. J. Ethnopharmacol. 2015, 159, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Roy, M.; Chakraborti, A.S. Ameliorative effects of glycyrrhizin on streptozotocin-induced diabetes in rats. J. Pharm. Pharmacol. 2011, 63, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.B.; Akram, M.; Muhammad Asif, H.; Qayyum, I.; Hashmi, A.M.; Munir, N.; Khan, F.S.; Riaz, M.; Ahmad, S. Antihyperglycemic activity of hydroalcoholic extracts of selective medicinal plants Curcuma longa, Lavandula stoechas, Aegle marmelos, and Glycyrrhiza glabra and their polyherbal preparation in Alloxan-induced diabetic mice. Dose Response 2019, 17, 1559325819852503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takii, H.; Kometani, T.; Nishimura, T.; Nakae, T.; Okada, S.; Fushiki, T. Antidiabetic effect of glycyrrhizin in genetically diabetic KK-Aymice. Biol. Pharm. Bull. 2001, 24, 484–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, W.Y.; Chia, Y.Y.; Liong, S.Y.; Ton, S.H.; Kadir, K.A.; Husain, S.N. Lipoprotein lipase expression, serum lipid and tissue lipid deposition in orally-administered glycyrrhizic acid-treated rats. Lipids Health Dis. 2009, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Choh, L. 11ß-Hydroxysteroid Dehydrogenase Type 1 and 2 and HOMAIR in Orally-Administered Glycyrrhizic Acid; Monash University Malaysia: Subang Jaya, Malaysia, 2008. [Google Scholar]
- Eu, C.H.; Lim, W.Y.; Ton, S.H.; bin Abdul Kadir, K. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis. 2010, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Yaw, H.P.; Ton, S.H.; Kadir, K.A. Glycyrrhizic Acid as the Modulator of 11β -hydroxysteroid dehydrogenase (Type 1 and 2) in rats under different physiological conditions in relation to the metabolic syndrome. J. Diabetes Metab. 2015, 6, 522. [Google Scholar]
- Alaa Eldin, A.H. Curcuma longa, Glycyrrhiza glabra Linn. and Moringa oleifera ameliorate diclofenac-induced hepatotoxicity in rats. Am. J. Pharm. Toxicol. 2007, 2, 80–88. [Google Scholar]
- Jeong, H.G.; You, H.J.; Park, S.J.; Moon, A.R.; Chung, Y.C.; Kang, S.K.; Chun, H.K. Hepatoprotective effects of 18β- glycyrrhetinic acid on carbon tetrachloride-induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharm. Res. 2002, 46, 221–227. [Google Scholar] [CrossRef]
- Xu-ying, W.; Ming, L.; Xiao-dong, L.; Ping, H. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. J. Chemico-Biological. Interact. 2009, 181, 15–19. [Google Scholar]
- Shi, Y.; Wu, D.; Sun, Z.; Yang, J.; Chai, H.; Tang, L.; Guo, Y. Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra. Phytother. Res. 2012, 26, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.A.; Patil, R.B.; Ghode, P.D.; Patole, V.; Pachauri, D.; Sherief, S.H. Aphrodisiac activity of aqueous extract of Glycyrrhiza glabra in male wistar rats. WJPR 2012, 1, 371–378. [Google Scholar]
- Shin, Y.W.; Bae, E.A.; Lee, B.; Lee, S.H.; Kim, J.A.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Med. 2007, 73, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Fiore, C.; Mattarello, M.J.; Bielenberg, J.; Palermo, M. History of the endocrine effects of licorice. Exp. Clin. Endocrinol. Diabetes 2002, 110, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Parle, M.; Dhingra, D.; Kulkarni, S.K. Neuromodulators of learning and memory. Asia Pac. J. Pharm. 2004, 16, 89–99. [Google Scholar]
- Tewari, D.; Stankiewicz, A.M.; Mocan, A.; Sah, A.N.; Tzvetkov, N.T.; Huminiecki, L.; Horbańczuk, J.O.; Atanasov, A.G. Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front. Aging Neurosci. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curreli, F.; Friedman, K.; Flore, A.O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering P53 mediated apoptosis in transformed B lymphocytes. J. Clin. Investig. 2005, 115, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Kumada, H. Long-term treatment of chronic hepatitis C with glycyrrhizin [stronger neo-minophagen C (SNMC)] for preventing liver cirrhosis and hepatocellular carcinoma. Oncology 2002, 62, 94–100. [Google Scholar] [CrossRef]
- Armanini, D.; Karbowiak, I.; Funder, J.W. Affinity of liquorice derivatives for mineralocorticoid and glucocorticoid receptors. Clin. Endocrinol. (Oxf.) 1983, 19, 609–612. [Google Scholar] [CrossRef]
- Tomlinson, J.W.; Walker, E.A.; Bujalska, I.J.; Draper, N.; Lavery, G.G.; Cooper, M.S.; Hewison, M.; Stewart, P.M. 11beta-hydroxysteroid dehydrogenase type 1: A tissue-specific regulator of glucocorticoid response. Endocr. Rev. 2004, 25, 831–866. [Google Scholar] [CrossRef]
- Chapman, K.; Holmes, M.; Seckl, J. 11β-hydroxysteroid dehydrogenases: Intracellular gate-keepers of tissue glucocorticoid action. Physiol. Rev. 2013, 93, 1139–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, C.; Bordin, L.; Pellati, D.; Armanini, D.; Clari, G. Effect of glycyrrhetinic acid on membrane band 3 in human erythrocytes. Arch. Biochem. Biophys. 2008, 479, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Andrisani, A.; Bordin, L.; Sabbadin, C. Spironolactone in the treatment of polycystic ovary syndrome. Expert. Opin. Pharmacother. 2016, 17, 1713–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armanini, D.; Castello, R.; Scaroni, C.; Bonanni, G.; Faccini, G.; Pellati, D.; Bertoldo, A.; Fiore, C.; Moghetti, P. Treatment of polycystic ovary syndrome with spironolactone plus licorice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2007, 13, 61–67. [Google Scholar] [CrossRef]
- Ton, S.H.; Chandramouli, C.; Bak, K. Glycyrrhizic acid: Biological effects on glucose and lipid metabolism. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3803–3826. [Google Scholar]
- Ploeger, B.T.; Mensinga, A.; Sips, W.; Seinen, J.; Meulenbelt, J.; DeJongh, J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab. Rev. 2001, 33, 125–147. [Google Scholar] [CrossRef]
- Nayak, C.; Singh, V.; Singh, K. Glycyrrhiza glabra-A multicentric clinical verification study. Indian J. Res. Homeopath. 2010, 4, 22–26. [Google Scholar]
- Adeneye, A.A. Subchronic and chronic toxicities of African medicinal plants. Toxicol. Surv. Afr. Med. Plants 2014, 99–133. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Glycyrrhiza glabra: A phytochemical and pharmacological review. J. Pharm. 2018, 8, 1–17. [Google Scholar]
- Vispute, S.; Khopade, A. Glycyrrhiza glabra Linn–Klitaka: A review. Inter. J. Pharma Bio Sci. 2011, 2, 42–51. [Google Scholar]
- Batiha, G.-S.; Alkazmi, L.M.; Nadwa, E.H.; Rashwan, E.K.; Beshbishy, A.M. Physostigmine: A plant alkaloid isolated from Physostigma venenosum: A review on pharmacokinetics, pharmacological and toxicological activities. J. Drug Deliv. Therap. 2020, 10, 187–190. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.-S.; Beshbishy, A.M.; Adeyemi, O.S.; Nadwa, E.H.; Rashwan, E.M.; Alkazmi, L.M.; Elkelish, A.A.; Igarashi, I. Phytochemical screening and antiprotozoal effects of the methanolic Berberis vulgaris and acetonic Rhus coriaria extracts. Molecules 2020, 25, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Taxonomy | |
|---|---|
| Realm | Plantae |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Order | Fabales |
| Family | Fabaceae |
| Genus | Glycyrrhiza |
| Species | Glycyrrhiza glabra L. |
| Compound | IUPAC Name | Chemical Formula | Compound | IUPAC Name | Chemical Formula |
|---|---|---|---|---|---|
| Glycyrrhizin | (3β,20β)-20-carboxy-11-oxo-30-norolean-12-en-3-yl 2-O-β-D-glucopyranuronosyl-α-D-glucopyranosiduronic acid | C42H62O16 | Glabridin | 4-[(3R)-8,8-Dimethyl-3,4-dihydro-2H,8H-pyrano [2,3-f]chromen-3-yl]-1,3-benzenediol | C20H20O4 |
| Glycyrrhizic acid | (2S,3S,4S,5R,6R)-6-[(2S,3R,4S,5S,6S)-2-[[(3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-carboxy-4,4,6a,6b,8a,11,14b-heptamethyl-14-oxo-2,3,4a,5,6,7,8,9,10,12,12a,14a-dodecahydro-1H-picen-3-yl]oxy]-6-carboxy-4,5-dihydroxyoxan-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid | C42H62O16 | Glabrene | 8-(7-hydroxy-2H-chromen-3-yl)-2,2-dimethylchromen-5-ol | C20H18O4 |
| Isoliquiritigenin | (E)-1-(2,4-Dihydroxyphenyl)-3-(4-hydroxyphenyl)prop-2-en-1-one | C15H12O4 | Licocoumarin A | 3-[2,4-dihydroxy-3-(3-methylbut-2-enyl)phenyl]-7-hydroxy-8-(3-methylbut-2-enyl)chromen-2-one | C25H26O5 |
| Licochalcone A | (E)-3-[4-Hydroxy-2-methoxy-5-(2-methylbut-3-en-2-yl)phenyl]-1-(4-hydroxyphenyl)prop-2-en-1-one | C21H22O4 | 18-β-Glycyrrhetinic acid | (2R,4aS,6aS,6bR,8aR,10S,12aS,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,14b-dodecahydro-1H-picene-2-carboxylic acid | C30H46O4 |
| Liquiritigenin | (2S)-7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-chromen-4-one | C15H12O4 | Liquiritin | (2S)-7-hydroxy-2-[4-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-2,3-dihydrochromen-4-one | C21H22O9 |
| Prenyllicoflavone A | 7-Hydroxy-2-[4-hydroxy-3-(3-methyl-2-buten-1-yl)phenyl]-6-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one | C25H26O4 | Kanzonol R | 3-[2-hydroxy-4-methoxy-3-(3-methylbut-2-enyl)phenyl]-5-methoxy-3,4-dihydro-2H-chromen-7-ol | C22H26O5 |
| α-Terpineol | 2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol | C10H18O | Glisoflavone | 3-[3,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-7-hydroxy-5-methoxychromen-4-one | C21H20O6 |
| Shinpterocarpin | (2R,10R)-17,17-dimethyl-3,12,18-trioxapentacyclo[11.8.0.02,10.04,9.014,19]henicosa-1(13),4(9),5,7,14(19),15,20-heptaen-6-ol | C20H18O4 | Isoangustone A | 3-[3,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-5,7-dihydroxy-6-(3-methylbut-2-enyl)chromen-4-one | C25H26O6 |
| 1-Methoxyficifolinol | (6aR,11aR)-1-methoxy-2,8-bis(3-methylbut-2-enyl)-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromene-3,9-diol | C26H30O5 | 2,3-Butanediol | Butane-2,3-diol | C4H10O2 |
| Licoriphenone | 1-(2,4-dihydroxyphenyl)-2-[6-hydroxy-2,4-dimethoxy-3-(3-methylbut-2-enyl)phenyl]ethanone | C21H24O6 | Semilicoisoflavone B | 5,7-dihydroxy-3-(8-hydroxy-2,2-dimethylchromen-6-yl)chromen-4-one | C20H16O6 |
| Licoarylcoumarin | 3-(2,4-dihydroxyphenyl)-7-hydroxy-5-methoxy-8-(2-methylbut-3-en-2-yl)chromen-2-one | C21H20O6 | Licopyranocoumarin | 7-(2,4-dihydroxyphenyl)-2-(hydroxymethyl)-5-methoxy-2-methyl-3,4-dihydropyrano[3,2-g]chromen-8-one | C21H20O7 |
| Furfuraldehyde | Furan-2-carbaldehyde | C5H4O2 | Tetramethyl pyrazine | tetramethyl pyrazine-2,3,5,6-tetracarboxylate | C12H12N2O8 |
| Activities | Chemical Component | Category | References |
|---|---|---|---|
| Antiulcer | Glycyrrhizic acid and glabridin, glabrene | Triterpenoid saponin and flavonoid | [50] |
| Antimycobacterial | Isoliquiritigenin | Flavonoid | [42] |
| Uterine relaxant and analgesic | Licocoumarin, licochalcone, isoliquiritigenin, and glabridin | Coumarin and flavonoids | [97] |
| Antioxidant | Glabridin | Flavonoid | [51,52] |
| Memory-enhancing activity | 18-β-glycyrrhetinic acid | Triterpenoid | [82] |
| Corticosteroidal activity | Liquiritigenin, glycyrrhizin, and 18-β-glycyrrhetinic acid | Flavonoid and triterpenoid saponin | [54] |
| Antiallergic | Glycyrrhizin | Triterpenoid saponin | [98] |
| Hepatoprotective | Liquiritoside and glycyrrhetic A | Flavonoid and triterpenoid saponin | [95] |
| Anti-inflammatory | Glycyrrhizin and glycyrrhetic A | Flavonoid | [26,60] |
| Anticancer | Licochalcone A | Flavonoid | [66] |
| Antimalarial | Glycyrrhizin, licochalcone, glycyrrhetinic acid | Flavonoid and triterpenoid | [47] |
| Antiviral activity | Glycyrrhizin and 18-β-glycyrrhetinic acid | Triterpenoid saponin | [45,46] |
| Antihyperglycemic | Glycyrrhizin | Triterpenoid saponin | [87] |
| Antitussive activity | Isoliquiritigenin and glycyrrhizin | Flavonoid and triterpenoid saponin | [35] |
| Immunostimulating activity | Glycyrrhizin | Triterpenoid saponin | [82] |
| Anti-HIV | Glycyrrhizin | Triterpenoid saponin | [45] |
| Muscle relaxant | Glabridin | Flavonoid | [71] |
| Antimicrobial | Liquiritigenin and glabrene | Flavonoid | [25,41] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; M. Abdel-Daim, M.; Prasad Devkota, H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030352
El-Saber Batiha G, Magdy Beshbishy A, El-Mleeh A, M. Abdel-Daim M, Prasad Devkota H. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules. 2020; 10(3):352. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030352
Chicago/Turabian StyleEl-Saber Batiha, Gaber, Amany Magdy Beshbishy, Amany El-Mleeh, Mohamed M. Abdel-Daim, and Hari Prasad Devkota. 2020. "Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae)" Biomolecules 10, no. 3: 352. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030352