Biological Evaluation and Docking Studies of New Carbamate, Thiocarbamate, and Hydrazide Analogues of Acyl Homoserine Lactones as Vibrio fischeri-Quorum Sensing Modulators
Abstract
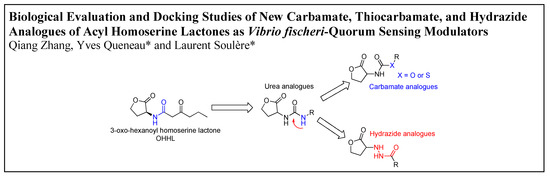
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. General Procedure for Route A
2.1.2. General Procedure for Route B
2.1.3. General Procedure for Route C (Hydrazides)
2.2. Biological Evaluation
2.3. Molecular Modeling
3. Results and Discussion
3.1. Synthesis
3.2. Biological Evaluation
3.3. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fuqua, C.; Greenberg, E.P. Self perception in bacteria: Quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1998, 1, 183–189. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G.; Kumar, A. Bacterial quorum sensing: Circuits and applications. Antonie Van Leeuwenhoek 2014, 105, 289–305. [Google Scholar] [CrossRef] [PubMed]
- Geske, G.D.; O’Neill, J.C.; Blackwell, H.E. Expanding dialogues: From natural autoinducers to non-natural analogues that modulate quorum sensing in Gram-negative bacteria. Chem. Soc. Rev. 2008, 37, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.E.; Chen, L. Structural basis of acyl-homoserine lactone-dependent signaling. Chem. Rev. 2011, 111, 68–85. [Google Scholar] [CrossRef] [Green Version]
- Brackman, G.; Coenye, T. Quorum sensing inhibitors as anti-biofilm agents. Curr. Pharm. Des. 2015, 21, 5–11. [Google Scholar] [CrossRef]
- Galloway, W.R.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef]
- LaSarre, B.; Federle, M.J. Exploiting quorum sensing to confuse bacterial pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [Green Version]
- Reuter, K.; Steinbach, A.; Helms, V. Interfering with Bacterial Quorum Sensing. Perspect. Med. Chem. 2016, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.M.; Queneau, Y.; Soulere, L.; von Bodman, S.; Doutheau, A. Mechanisms and synthetic modulators of AHL-dependent gene regulation. Chem. Rev. 2011, 111, 4–27. [Google Scholar] [CrossRef]
- Tay, S.B.; Yew, W.S. Development of quorum-based anti-virulence therapeutics targeting Gram-negative bacterial pathogens. Int. J. Mol. Sci. 2013, 14, 16570–16599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ma, S. Small molecules modulating AHL-based quorum sensing to attenuate bacteria virulence and biofilms as promising antimicrobial drugs. Curr. Med. Chem. 2014, 21, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; Castang, S.; Estephane, J.; Soulere, L.; Deshayes, C.; Chantegrel, B.; Nasser, W.; Queneau, Y.; Reverchon, S.; Doutheau, A. Synthesis and biological evaluation of homoserine lactone derived ureas as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. 2006, 14, 4781–4791. [Google Scholar] [CrossRef] [PubMed]
- Frezza, M.; Soulere, L.; Reverchon, S.; Guiliani, N.; Jerez, C.; Queneau, Y.; Doutheau, A. Synthetic homoserine lactone-derived sulfonylureas as inhibitors of Vibrio fischeri quorum sensing regulator. Bioorg. Med. Chem. 2008, 16, 3550–3556. [Google Scholar] [CrossRef]
- Castang, S.; Chantegrel, B.; Deshayes, C.; Dolmazon, R.; Gouet, P.; Haser, R.; Reverchon, S.; Nasser, W.; Hugouvieux-Cotte-Pattat, N.; Doutheau, A. N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg. Med. Chem. Lett. 2004, 14, 5145–5149. [Google Scholar] [CrossRef]
- Boukraa, M.; Sabbah, M.; Soulere, L.; El Efrit, M.L.; Queneau, Y.; Doutheau, A. AHL-dependent quorum sensing inhibition: Synthesis and biological evaluation of alpha-(N-alkyl-carboxamide)-gamma-butyrolactones and alpha-(N-alkyl-sulfonamide)-gamma-butyrolactones. Bioorg. Med. Chem. Lett. 2011, 21, 6876–6879. [Google Scholar] [CrossRef]
- Sabbah, M.; Fontaine, F.; Grand, L.; Boukraa, M.; Efrit, M.L.; Doutheau, A.; Soulere, L.; Queneau, Y. Synthesis and biological evaluation of new N-acyl-homoserine-lactone analogues, based on triazole and tetrazole scaffolds, acting as LuxR-dependent quorum sensing modulators. Bioorg. Med. Chem. 2012, 20, 4727–4736. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Beaber, J.W.; More, M.I.; Fuqua, C.; Eberhard, A.; Winans, S.C. Analogs of the autoinducer 3-oxooctanoyl-homoserine lactone strongly inhibit activity of the TraR protein of Agrobacterium tumefaciens. J. Bacteriol. 1998, 180, 5398–5405. [Google Scholar] [CrossRef] [Green Version]
- Persson, T.; Hansen, T.H.; Rasmussen, T.B.; Skinderso, M.E.; Givskov, M.; Nielsen, J. Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic. Org. Biomol. Chem. 2005, 3, 253–262. [Google Scholar] [CrossRef]
- Uzar, H.C. Improved Synthesis of L-Homoserine Derivatives from L-Aspartic Acid. Synthesis 1991, 1991, 526–528. [Google Scholar] [CrossRef]
- Lall, M.S.; Karvellas, C.; Vederas, J.C. Beta-lactones as a new class of cysteine proteinase inhibitors: Inhibition of hepatitis A virus 3C proteinase by N-Cbz-serine beta-lactone. Org. Lett. 1999, 1, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, A.; Kratz, F. 2,4-bis(hydroxymethyl)aniline as a building block for oligomers with self-eliminating and multiple release properties. J. Org. Chem. 2008, 73, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.E.; Adlington, R.M.; Moss, N. Synthetic Approaches to 2-Methoxycysteine containing Peptides. The conversion of [(5S)-5-amino-5-carboxy-2-oxapentanoyl]-2-methoxy-(2S)-cysteinyl-(2R)-valine into 10-Oxa-6α-methoxyisopenicillin N by the Enzyme Isopenicillin N Synthase. Tetrahedron 1989, 45, 2841–2856. [Google Scholar] [CrossRef]
- Saha, A.; Kumar, R.; Devakumar, C. Development and assessment of green synthesis of hydrazides. Indian J. Chem. B 2010, 49, 526–531. [Google Scholar]
- Demange, L.; Boeglin, D.; Moulin, A.; Mousseaux, D.; Ryan, J.; Berge, G.; Gagne, D.; Heitz, A.; Perrissoud, D.; Locatelli, V.; et al. Synthesis and pharmacological in vitro and in vivo evaluations of novel triazole derivatives as ligands of the ghrelin receptor. 1. J. Med. Chem. 2007, 50, 1939–1957. [Google Scholar] [CrossRef]
- Geske, G.D.; O’Neill, J.C.; Blackwell, H.E. N-phenylacetanoyl-L-homoserine lactones can strongly antagonize or superagonize quorum sensing in Vibrio fischeri. ACS Chem. Biol. 2007, 2, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Syrpas, M.; Ruysbergh, E.; Stevens, C.V.; De Kimpe, N.; Mangelinckx, S. Synthesis and biological evaluation of novel N-alpha-haloacylated homoserine lactones as quorum sensing modulators. Beilstein J. Org. Chem. 2014, 10, 2539–2549. [Google Scholar] [CrossRef] [Green Version]
- Soulere, L.; Frezza, M.; Queneau, Y.; Doutheau, A. Exploring the active site of acyl homoserine lactones-dependent transcriptional regulators with bacterial quorum sensing modulators using molecular mechanics and docking studies. J. Mol. Graph. Model. 2007, 26, 581–590. [Google Scholar] [CrossRef]
- Estephane, J.; Dauvergne, J.; Soulere, L.; Reverchon, S.; Queneau, Y.; Doutheau, A. N-Acyl-3-amino-5H-furanone derivatives as new inhibitors of LuxR-dependent quorum sensing: Synthesis, biological evaluation and binding mode study. Bioorg. Med. Chem. Lett. 2008, 18, 4321–4324. [Google Scholar] [CrossRef]
- Soulere, L.; Queneau, Y. Conformational and docking studies of acyl homoserine lactones as a robust method to investigate bioactive conformations. Comput. Biol. Chem. 2019, 79, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Thompson, M.A. ArgusLaB Software, Version 4.0.1; Planetaria Software LLC: Seattle, WA, USA, 2004. [Google Scholar]
- Rahmathullah, S.M.; Hall, J.E.; Bender, B.C.; McCurdy, D.R.; Tidwell, R.R.; Boykin, D.W. Prodrugs for amidines: Synthesis and anti-Pneumocystis carinii activity of carbamates of 2,5-bis(4-amidinophenyl)furan. J. Med. Chem. 1999, 42, 3994–4000. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, S.; Chantegrel, B.; Deshayes, C.; Doutheau, A.; Cotte-Pattat, N. New synthetic analogues of N-acyl homoserine lactones as agonists or antagonists of transcriptional regulators involved in bacterial quorum sensing. Bioorg. Med. Chem. Lett. 2002, 12, 1153–1157. [Google Scholar] [CrossRef]
- Li, S.Z.; Xu, R.; Ahmar, M.; Goux-Henry, C.; Queneau, Y.; Soulere, L. Influence of the d/l configuration of N-acyl-homoserine lactones (AHLs) and analogues on their Lux-R dependent quorum sensing activity. Bioorg. Chem. 2018, 77, 215–222. [Google Scholar] [CrossRef]
- Gregoret, L.M.; Rader, S.D.; Fletterick, R.J.; Cohen, F.E. Hydrogen bonds involving sulfur atoms in proteins. Proteins 1991, 9, 99–107. [Google Scholar] [CrossRef]
- Ahumedo, M.; Drosos, J.C.; Vivas-Reyes, R. Application of molecular docking and ONIOM methods for the description of interactions between anti-quorum sensing active (AHL) analogues and the Pseudomonas aeruginosa LasR binding site. Mol. Biosyst. 2014, 10, 1162–1171. [Google Scholar] [CrossRef]




| Compounds | Predicted logP | IC50 (µM) |
|---|---|---|
| Butyl (2) | 0.65 | 94 (±1) |
| Hexyl (4) | 1.19 | 73 (±3) |
| Bn (5) | 1.14 | 86 (±3) |
| 4-BrBn (6) | 1.97 | 64 (±4) |
| 4-NO2Bn (7) | 0.51 | 22 (±3) |
| ButylS (9) | 1.22 | 45 (±7) |
| BnS (10) | 1.71 | 64 (±3) |
| 4-NO2BnS (11) | 1.17 | 23 (±4) |
| Compounds | Trp66 | Asp79 | Tyr62 | Tyr70 | Lys178 |
|---|---|---|---|---|---|
| OHHL (natural ligand) | +2.34 (C=O lactone) | +3.01 (NH amide) | +3.01 (C=O amide) | - | - |
| Ethyl (1 agonist) | +2.44 (C=O lactone) | +3.0 (NH carbamate) | +2.91 (C=O carbamate) | - | - |
| Butyl (2) | +2.20 (C=O lactone) | +2.92 (NH carbamate) | - | +2.97 (O carbamate) | - |
| Hexyl (4) | +2.22 (C=O lactone) | +2.98 (NH amide) | - | +2.61 (O carbamate) | - |
| Bn (5) | +2.89 (C=O lactone) | +2.98 (NH carbamate) | - | +3.01 (O carbamate) | - |
| 4-BrBn (6) | +2.35 (C=O lactone) | +2.95 (NH carbamate) | +3.01 (C=O carbamate) | +3.32 (O carbamate) | - |
| 4-NO2Bn (7) | +2.07 (C=O lactone) | +2.99 (NH carbamate) | - | +3.04 (O carbamate) | ++ 2.22 and 3.05 (NO2) |
| ButylS (9) | +2.63 (C=O lactone) | +3.00 (NH carbamate) | +3.09 (C=O carbamate) | +3.19 (S carbamate) | - |
| BnS (10) | +2.89 (C=O lactone) | +2.98 (NH carbamate) | +3.13 (C=O carbamate) | +3.08 (S carbamate) | - |
| 4-NO2BnS (11) | +2.12 (C=O lactone) | +3.14 (NH carbamate) | - | +3.25 (S carbamate) | ++ 1.96 and 3.85 (NO2) |
| Hydrazide (12) | +2.45 (C=O lactone) | +2.94 (NH hydrazide) | - | +2.21 (C=O hydrazide) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Queneau, Y.; Soulère, L. Biological Evaluation and Docking Studies of New Carbamate, Thiocarbamate, and Hydrazide Analogues of Acyl Homoserine Lactones as Vibrio fischeri-Quorum Sensing Modulators. Biomolecules 2020, 10, 455. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030455
Zhang Q, Queneau Y, Soulère L. Biological Evaluation and Docking Studies of New Carbamate, Thiocarbamate, and Hydrazide Analogues of Acyl Homoserine Lactones as Vibrio fischeri-Quorum Sensing Modulators. Biomolecules. 2020; 10(3):455. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030455
Chicago/Turabian StyleZhang, Qiang, Yves Queneau, and Laurent Soulère. 2020. "Biological Evaluation and Docking Studies of New Carbamate, Thiocarbamate, and Hydrazide Analogues of Acyl Homoserine Lactones as Vibrio fischeri-Quorum Sensing Modulators" Biomolecules 10, no. 3: 455. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10030455