Bioconversion of Biologically Active Indole Derivatives with Indole-3-Acetic Acid-Degrading Enzymes from Caballeronia glathei DSM50014
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Bacterial Strains and Growth Conditions
2.2. Cloning and Expression of Iac Genes
2.3. Whole-Cell Bioconversion
2.4. Analytical Techniques
2.5. Utilization of H218O
3. Results
3.1. Identification of Caballeronia Glathei DSM50014 as a Biodegrader of IAA
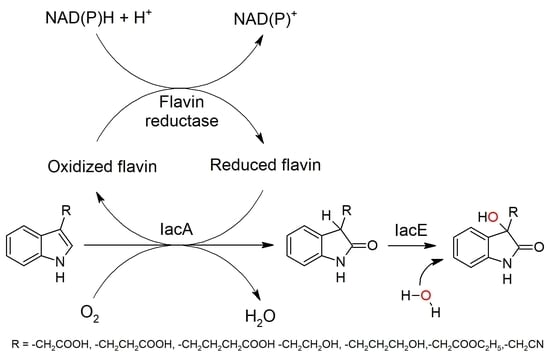
3.2. IacA- and IacE-Catalyzed Reactions in IAA Degradation
3.3. Substrate Scope of IacA and IacE Proteins
3.4. Oxygen Incorporated into DOAA is Derived from Water
4. Discussion
4.1. Involvement and Role of IacB in IAA Biodegradation
4.2. The Role of Other Iac Proteins and Analogies with Indole Biodegradation
4.3. Perspectives of the Bioconversion of IAA Homologs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.-H.; Wood, T.K.; Lee, J. Roles of Indole as an Interspecies and Interkingdom Signaling Molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Young, K.D. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 2013, 159, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, W. Indole: A signaling molecule or a mere metabolic byproduct that alters bacterial physiology at a high concentration? J. Microbiol. 2015, 53, 421–428. [Google Scholar] [CrossRef]
- Zarkan, A.; Caño-Muñiz, S.; Zhu, J.; Nahas, K.A.; Cama, J.; Keyser, U.F.; Summers, D.K. Indole Pulse Signalling Regulates the Cytoplasmic pH of E. coli in a Memory-Like Manner. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, E.; Giraud, E.; Baucheron, S.; Yamasaki, S.; Wiedemann, A.; Okamoto, K.; Takagi, T.; Yamaguchi, A.; Cloeckaert, A.; Nishino, K. Effects of indole on drug resistance and virulence of Salmonella enterica serovar Typhimurium revealed by genome-wide analyses. Gut Pathog. 2012, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sperandio, V. Indole Signaling at the Host-Microbiota-Pathogen Interface. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Kim, Y.-G.; Kim, M.; Kim, E.; Choi, H.; Kim, Y.; Lee, J. Indole-associated predator-prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 2017, 19, 1776–1790. [Google Scholar] [CrossRef] [PubMed]
- Huc, T.; Konop, M.; Onyszkiewicz, M.; Podsadni, P.; Szczepańska, A.; Turło, J.; Ufnal, M. Colonic indole, gut bacteria metabolite of tryptophan, increases portal blood pressure in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R646–R655. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomainen, M.; Lindström, J.; Lehtonen, M.; Auriola, S.; Pihlajamäki, J.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M.; de Mello, V.D.; Hanhineva, K. Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 2018, 8, 1–5. [Google Scholar] [CrossRef]
- de Mello, V.D.; Paananen, J.; Lindström, J.; Lankinen, M.A.; Shi, L.; Kuusisto, J.; Pihlajamäki, J.; Auriola, S.; Lehtonen, M.; Rolandsson, O.; et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci. Rep. 2017, 7, 46337. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Xin, F.-Z.; Xue, Y.; Hu, Z.; Han, Y.; Ma, F.; Zhou, D.; Liu, X.-L.; Cui, A.; Liu, Z.; et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.-Y.; Lin, C.-J.; Pan, H.-C.; Lee, C.-C.; Lu, S.-C.; Hsieh, Y.-T.; Huang, S.-Y.; Huang, H.-Y. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clin. Nutr. 2019, 38, 2945–2948. [Google Scholar] [CrossRef]
- Konopelski, P.; Konop, M.; Gawrys-Kopczynska, M.; Podsadni, P.; Szczepanska, A.; Ufnal, M. Indole-3-Propionic Acid, a Tryptophan-Derived Bacterial Metabolite, Reduces Weight Gain in Rats. Nutrients 2019, 11, 591. [Google Scholar] [CrossRef] [Green Version]
- Negatu, D.A.; Liu, J.J.J.; Zimmerman, M.; Kaya, F.; Dartois, V.; Aldrich, C.C.; Gengenbacher, M.; Dick, T. Whole-Cell Screen of Fragment Library Identifies Gut Microbiota Metabolite Indole Propionic Acid as Antitubercular. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Negatu, D.A.; Yamada, Y.; Xi, Y.; Go, M.L.; Zimmerman, M.; Ganapathy, U.; Dartois, V.; Gengenbacher, M.; Dick, T. Gut Microbiota Metabolite Indole Propionic Acid Targets Tryptophan Biosynthesis in Mycobacterium tuberculosis. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Zhang, X.; Qu, Y. Biodegradation and Biotransformation of Indole: Advances and Perspectives. Front Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Leveau, J.H.J.; Lindow, S.E. Utilization of the Plant Hormone Indole-3-Acetic Acid for Growth by Pseudomonas putida Strain 1290. Appl. Environ. Microbiol. 2005, 71, 2365–2371. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.-H.; Chen, H.-P.; Huang, J.-H.; Liu, T.-T.; Lin, T.-K.; Wang, S.-J.; Tseng, C.-H.; Shu, H.-Y. Identification and characterization of an indigo-producing oxygenase involved in indole 3-acetic acid utilization by Acinetobacter baumannii. Antonie van Leeuwenhoek 2012, 101, 881–890. [Google Scholar] [CrossRef]
- Donoso, R.; Leiva-Novoa, P.; Zúñiga, A.; Timmermann, T.; Recabarren-Gajardo, G.; González, B. Biochemical and Genetic Bases of Indole-3-Acetic Acid (Auxin Phytohormone) Degradation by the Plant-Growth-Promoting Rhizobacterium Paraburkholderia phytofirmans PsJN. Appl. Environ. Microbiol. 2016, 83. [Google Scholar] [CrossRef] [Green Version]
- Greenhut, I.V.; Slezak, B.L.; Leveau, J.H.J. iac Gene Expression in the Indole-3-Acetic Acid-Degrading Soil Bacterium Enterobacter soli LF7. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [Green Version]
- Epstein, E.; Ludwig-Müller, J. Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Strader, L.C.; Bartel, B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant. 2011, 4, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Zolman, B.K.; Yoder, A.; Bartel, B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 2000, 156, 1323–1337. [Google Scholar]
- Leveau, J.H.J.; Gerards, S. Discovery of a bacterial gene cluster for catabolism of the plant hormone indole 3-acetic acid. FEMS Microbiol. Ecol. 2008, 65, 238–250. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.C.; Greenhut, I.V.; Leveau, J.H.J. Functional Characterization of the Bacterial iac Genes for Degradation of the Plant Hormone Indole-3-Acetic Acid. J. Chem. Ecol. 2013, 39, 942–951. [Google Scholar] [CrossRef]
- Heine, T.; Großmann, C.; Hofmann, S.; Tischler, D. Indigoid dyes by group E monooxygenases: Mechanism and biocatalysis. Biol. Chem. 2019, 400, 939–950. [Google Scholar] [CrossRef]
- Kavanagh, K.L.; Jörnvall, H.; Persson, B.; Oppermann, U. Medium- and short-chain dehydrogenase/reductase gene and protein families. Cell Mol. Life Sci. 2008, 65, 3895–3906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Wei, Y.; Liu, X.; Zhou, Y.; Jiang, L.; Yin, J.; Wang, F.; Hu, Y.; Urs, A.N.N.; Liu, Y.; et al. Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zolg, W.; Ottow, J.C. Pseudomonas glathei sp. nov., a new nitrogen-scavening rod isolated from acid lateritic relicts in Germany. J. Comp. Neurol. 1975, 164, 287–299. [Google Scholar]
- Viallard, V.; Poirier, I.; Cournoyer, B.; Haurat, J.; Wiebkin, S.; Ophel-Keller, K.; Balandreau, J. Burkholderia graminis sp. nov., a rhizospheric Burkholderia species, and reassessment of [Pseudomonas] phenazinium, [Pseudomonas] pyrrocinia and [Pseudomonas] glathei as Burkholderia. Int. J. Syst. Bacteriol. 1998, 48, 549–563. [Google Scholar] [CrossRef]
- Sawana, A.; Adeolu, M.; Gupta, R.S. Molecular signatures and phylogenomic analysis of the genus Burkholderia: Proposal for division of this genus into the emended genus Burkholderia containing pathogenic organisms and a new genus Paraburkholderia gen. nov. harboring environmental species. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Dobritsa, A.P.; Samadpour, M. Transfer of eleven species of the genus Burkholderia to the genus Paraburkholderia and proposal of Caballeronia gen. nov. to accommodate twelve species of the genera Burkholderia and Paraburkholderia. Int. J. Syst. Evol. Microbiol. 2016, 66, 2836–2846. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Baldwin, A.; Dowson, C.G. Burkholderia cepacia complex bacteria: Opportunistic pathogens with important natural biology. J. Appl. Microbiol. 2008, 104, 1539–1551. [Google Scholar] [CrossRef]
- Stopnisek, N.; Zühlke, D.; Carlier, A.; Barberán, A.; Fierer, N.; Becher, D.; Riedel, K.; Eberl, L.; Weisskopf, L. Molecular mechanisms underlying the close association between soil Burkholderia and fungi. ISME J. 2016, 10, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-Y.; Li, C.-X.; Luo, X.-J.; Lai, Q.-L.; Xu, J.-H. Burkholderia jiangsuensis sp. nov., a methyl parathion degrading bacterium, isolated from methyl parathion contaminated soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 3247–3253. [Google Scholar] [CrossRef] [Green Version]
- Sambrook, J. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Sadauskas, M.; Vaitekūnas, J.; Gasparavičiūtė, R.; Meškys, R. Genetic and Biochemical Characterization of Indole Biodegradation in Acinetobacter sp. Strain O153. Appl. Environ. Microbiol. 2017, AEM.01453-17. [Google Scholar] [CrossRef] [Green Version]
- Petkevičius, V.; Vaitekūnas, J.; Tauraitė, D.; Stankevičiūtė, J.; Šarlauskas, J.; Čėnas, N.; Meškys, R. A Biocatalytic Synthesis of Heteroaromatic N-Oxides by Whole Cells of Escherichia coli Expressing the Multicomponent, Soluble Di-Iron Monooxygenase (SDIMO) PmlABCDEF. Adv. Synth. Catal. 2019, 361, 2456–2465. [Google Scholar] [CrossRef] [Green Version]
- Sadauskas, M.; Statkevičiūtė, R.; Vaitekūnas, J.; Petkevičius, V.; Časaitė, V.; Gasparavičiūtė, R.; Meškys, R. Enzymatic synthesis of novel water-soluble indigoid compounds. Dyes Pigments 2020, 173, 107882. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Qu, Y.; Ma, Q.; Liu, Z.; Wang, W.; Tang, H.; Zhou, J.; Xu, P. Unveiling the biotransformation mechanism of indole in a Cupriavidus sp. strain. Mol. Microbiol. 2017, 106, 905–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbers, M.M.E.; Montersino, S.; Westphal, A.H.; Tischler, D.; van Berkel, W.J.H. Flavin dependent monooxygenases. Arch. Biochem. Biophys. 2014, 544, 2–17. [Google Scholar] [CrossRef]
- Gräff, M.; Buchholz, P.C.F.; Stockinger, P.; Bommarius, B.; Bommarius, A.S.; Pleiss, J. The Short-chain Dehydrogenase/Reductase Engineering Database (SDRED): A classification and analysis system for a highly diverse enzyme family. Proteins 2019, 87, 443–451. [Google Scholar] [CrossRef]
- Kamano, Y.; Zhang, H.; Ichihara, Y.; Kizu, H.; Komiyama, K.; Pettit, G.R. Convolutamydine A, a novel bioactive hydroxyoxindole alkaloid from marine bryozoan Amathia convoluta. Tetrahedron Lett. 1995, 36, 2783–2784. [Google Scholar] [CrossRef]
- Kohno, J.; Koguchi, Y.; Nishio, M.; Nakao, K.; Kuroda, M.; Shimizu, R.; Ohnuki, T.; Komatsubara, S. Structures of TMC-95A−D: Novel Proteasome Inhibitors from Apiospora montagnei Sacc. TC 1093. J. Org. Chem. 2000, 65, 990–995. [Google Scholar] [CrossRef]
- Suzuki, H.; Morita, H.; Shiro, M.; Kobayashi, J. Celogentin K, a new cyclic peptide from the seeds of Celosia argentea and X-ray structure of moroidin. Tetrahedron 2004, 60, 2489–2495. [Google Scholar] [CrossRef]
- Kagata, T.; Saito, S.; Shigemori, H.; Ohsaki, A.; Ishiyama, H.; Kubota, T.; Kobayashi, J. Paratunamides A−D, Oxindole Alkaloids from Cinnamodendron axillare. J. Nat. Prod. 2006, 69, 1517–1521. [Google Scholar] [CrossRef]
- Khuzhaev, V.U.; Zhalolov, I.; Turgunov, K.K.; Tashkhodzhaev, B.; Levkovich, M.G.; Aripova, S.F.; Shashkov, A.S. Alkaloids from Arundo donax. XVII. Structure of the Dimeric Indole Alkaloid Arundaphine. Chem. Nat. Comp. 2004, 40, 269–272. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E. Indole Propionic Acid: A Small Molecule Links between Gut Microbiota and Tuberculosis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Zhang, S.; Liu, L.; Duan, W.; Wang, W. Organocatalytic Enantioselective Cross-Aldol Reactions of Aldehydes with Isatins: Formation of Two Contiguous Quaternary Centered 3-Substituted 3-Hydroxyindol-2-ones. Chem. Asian J. 2009, 4, 1664–1667. [Google Scholar] [CrossRef]
- Heine, T.; Van Berkel, W.J.H.; Gassner, G.; Van Pée, K.-H.; Tischler, D. Two-Component FAD-Dependent Monooxygenases: Current Knowledge and Biotechnological Opportunities. Biology 2018, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Persson, B.; Bray, J.E.; Bruford, E.; Dellaporta, S.L.; Favia, A.D.; Gonzalez Duarte, R.; Jörnvall, H.; Kallberg, Y.; Kavanagh, K.L.; Kedishvili, N.; et al. The SDR (Short-Chain Dehydrogenase/Reductase and Related Enzymes) Nomenclature Initiative. Chem. Biol. Interact. 2009, 178, 94–98. [Google Scholar] [CrossRef] [Green Version]







| Substrate | Structure | Activity | |
|---|---|---|---|
| IacA (Bioconversion Efficiency, %) | IacE | ||
| IAA |  | + (34 ± 8) | + |
| IPA |  | + (22 ± 5) | + |
| IBA |  | + (21 ± 5) | + |
| Indole |  | + (NA) | − |
| 3-(2-hydroxyethyl)indole |  | + (32 ± 7) | + |
| Ethyl-3-indole-acetate |  | + (19 ± 6) | + |
| 3-(3-hydroxypropyl)indole |  | + (37 ± 8) | + |
| 3-indoleacetonitrile |  | + (>90) | + |
| Indole-3-acetamide |  | + (28 ± 5) | − |
| Indole-3-lactic acid |  | + (43 ± 11) | − |
| Tryptamine |  | − | NA |
| Indole-3-acrycil acid |  | − | NA |
| Indole-3-carboxylic acid |  | − | NA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadauskas, M.; Statkevičiūtė, R.; Vaitekūnas, J.; Meškys, R. Bioconversion of Biologically Active Indole Derivatives with Indole-3-Acetic Acid-Degrading Enzymes from Caballeronia glathei DSM50014. Biomolecules 2020, 10, 663. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10040663
Sadauskas M, Statkevičiūtė R, Vaitekūnas J, Meškys R. Bioconversion of Biologically Active Indole Derivatives with Indole-3-Acetic Acid-Degrading Enzymes from Caballeronia glathei DSM50014. Biomolecules. 2020; 10(4):663. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10040663
Chicago/Turabian StyleSadauskas, Mikas, Roberta Statkevičiūtė, Justas Vaitekūnas, and Rolandas Meškys. 2020. "Bioconversion of Biologically Active Indole Derivatives with Indole-3-Acetic Acid-Degrading Enzymes from Caballeronia glathei DSM50014" Biomolecules 10, no. 4: 663. https://0-doi-org.brum.beds.ac.uk/10.3390/biom10040663