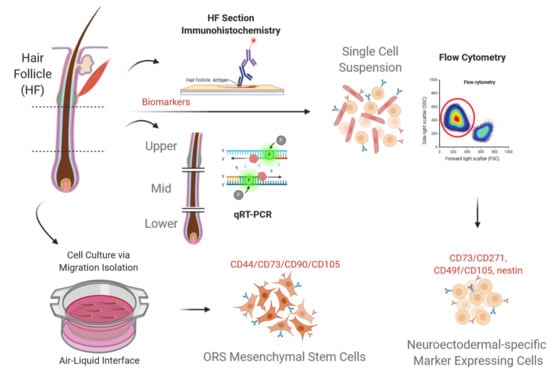
The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Histological Sections
2.2. Immunofluorescent Staining
2.3. Immunohistochemistry Staining
2.4. Flow Cytometry Analysis
2.5. Gene Expression
2.6. Statistical Analysis
3. Results
3.1. Immunostaining of Longitudinal Follicle Sections
3.2. Expression of Markers Analyzed by FCM
3.3. Gene Expression of Cell Type Markers Varies between Different Parts of the ORS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell–enriched human hair follicle bulge cells. J. Clin. Investig. 2006, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Amoh, Y.; Li, L.; Campillo, R.; Kawahara, K.; Katsuoka, K.; Penman, S.; Hoffman, R.M. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc. Natl. Acad. Sci. USA 2005, 102, 17734–17738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoh, Y.; Li, L.; Yang, M.; Moossa, A.R.; Katsuoka, K.; Penman, S.; Hoffman, R.M. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc. Natl. Acad. Sci. USA 2004, 101, 13291–13295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashiro, M.; Mii, S.; Aki, R.; Hamada, Y.; Arakawa, N.; Kawahara, K.; Hoffman, R.M.; Amoh, Y. From hair to heart: Nestin-expressing hair-follicle-associated pluripotent (HAP) stem cells differentiate to beating cardiac muscle cells. Cell Cycle 2015, 14, 2362–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoh, Y.; Li, L.; Katsuoka, K.; Penman, S.; Hoffman, R. Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl. Acad. Sci. USA 2005, 102, 5530–5534. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Fang, D.; Kumar, S.M.; Li, L.; Nguyen, T.K.; Acs, G.; Herlyn, M.; Xu, X. Isolation of a Novel Population of Multipotent Adult Stem Cells from Human Hair Follicles. Am. J. Pathol. 2006, 168, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Kumar, S.M.; Kossenkov, A.V.; Showe, L.; Xu, X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J. Invest. Dermatol. 2010, 130, 1227–1236. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xu, X. Isolation and culture of neural crest stem cells from human hair follicles. J. Vis. Exp. JoVE 2013, 74, 3194. [Google Scholar] [CrossRef]
- Levy, V.; Lindon, C.; Harfe, B.D.; Morgan, B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 2005, 9, 855–861. [Google Scholar] [CrossRef] [Green Version]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; van Es, J.H.; Barker, N.; van de Wetering, M.; van den Born, M.; Begthel, H.; Vries, R.G.; et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar] [CrossRef] [Green Version]
- Blanpain, C. Skin regeneration and repair. Nature 2010, 464, 686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Gao, Y.; Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Xu, H.; Liu, F.; et al. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Liu, F.; Wu, C.; Jiang, W.; Zhao, G.; Liu, L.; Bai, T.; Wang, L.; Jiang, Y.; Guo, L.; et al. Feasibility of human hair follicle-derived mesenchymal stem cells/CultiSpher®-G constructs in regenerative medicine. Cell Tissue Res. 2015, 362, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Masieri, F.F.; Schneider, M.; Kottek, T.; Hahnel, S.; Yamauchi, K.; Obradović, D.; Seon, J.-K.; Yun, S.J.; Ferrer, R.A.; et al. Autologous, Non-Invasively Available Mesenchymal Stem Cells from the Outer Root Sheath of Hair Follicle Are Obtainable by Migration from Plucked Hair Follicles and Expandable in Scalable Amounts. Cells 2020, 9, 2069. [Google Scholar] [CrossRef] [PubMed]
- Joulai Veijouye, S.; Yari, A.; Heidari, F.; Sajedi, N.; Ghoroghi Moghani, F.; Nobakht, M. Bulge Region as a Putative Hair Follicle Stem Cells Niche: A Brief Review. Iran. J. Public Health 2017, 46, 1167–1175. [Google Scholar] [PubMed]
- Schneider, M.; Dieckmann, C.; Rabe, K.; Simon, J.C.; Savkovic, V. Differentiating the Stem Cell Pool of Human Hair Follicle Outer Root Sheath into Functional Melanocytes. In Stem Cells and Tissue Repair. Methods in Molecular Biology (Methods and Protocols); Kioussi, C., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1210, pp. 203–227. [Google Scholar]
- Limat, A.; French, L.E.; Blal, L.; Saurat, J.H.; Hunziker, T.; Salomon, D. Organotypic cultures of autologous hair follicle keratinocytes for the treatment of recurrent leg ulcers. J. Am. Acad. Dermatol. 2003, 48, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Poblet, E.; Jiménez, F.; Godínez, J.M.; Pascual-Martín, A.; Izeta, A. The immunohistochemical expression of CD34 in human hair follicles: A comparative study with the bulge marker CK15. Clin. Exp. Dermatol. 2006, 31, 807–812. [Google Scholar] [CrossRef]
- Dieckmann, C.; Milkova, L.; Hunziker, T.; Emmendörffer, A.; Simon, J.C. Human melanocytes can be isolated, propagated and expanded from plucked anagen hair follicles. Exp. Dermatol. 2010, 19, 543–545. [Google Scholar] [CrossRef]
- Savkovic, V.; Dieckmann, C.; Milkova, L.; Simon, J.C. Improved method of differentiation, selection and amplification of human melanocytes from the hair follicle cell pool. Exp. Dermatol. 2012, 21, 948–950. [Google Scholar] [CrossRef]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006, 24, 928–935. [Google Scholar] [CrossRef]
- Krahl, D.; Sellheyer, K. The neuroepithelial stem cell protein nestin is a marker of the companion cell layer of the adult and developing human hair follicle. J. Br. J. Dermatol. 2009, 161, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mignone, J.; Yang, M.; Matic, M.; Penman, S.; Enikolopov, G.; Hoffman, R.M. Nestin expression in hair follicle sheath progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9958–9961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, W.-M.; Oro, A. SnapShot: Hair follicle stem cells. Cell 2011, 146, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. J. Lab. Investig. 2009, 89, 844. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Xu, X. Isolation and Culture of Neural Crest Stem Cells from Human Hair Follicles. In Multipotent Stem Cells of the Hair Follicle; Springer: Berlin/Heidelberg, Germany, 2016; pp. 49–55. [Google Scholar]
- Boxall, S.A.; Jones, E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int. 2012, 2012, 975871. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.; Carrillo-Gálvez, A.B.; García-Pérez, A.; Cobo, M.; Martín, F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE 2013, 8, e76979. [Google Scholar] [CrossRef]
- Yu, K.R.; Yang, S.R.; Jung, J.W.; Kim, H.; Ko, K.; Han, D.W.; Park, S.B.; Choi, S.W.; Kang, S.K.; Scholer, H.; et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells 2012, 30, 876–887. [Google Scholar] [CrossRef]
- Lee, R.H.; Seo, M.J.; Pulin, A.A.; Gregory, C.A.; Ylostalo, J.; Prockop, D.J. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 2009, 113, 816–826. [Google Scholar] [CrossRef] [Green Version]
- Altomonte, M.; Montagner, R.; Fonsatti, E.; Colizzi, F.; Cattarossi, I.; Brasoveanu, L.I.; Nicotra, M.R.; Cattelan, A.; Natali, P.G.; Maio, M. Expression and structural features of endoglin (CD105), a transforming growth factor beta1 and beta3 binding protein, in human melanoma. Br. J. Cancer 1996, 74, 1586–1591. [Google Scholar] [CrossRef] [Green Version]
- Narravula, S.; Lennon, P.F.; Mueller, B.U.; Colgan, S.P. Regulation of endothelial CD73 by adenosine: Paracrine pathway for enhanced endothelial barrier function. J. Immunol. 2000, 165, 5262–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Viejo, M.; Menéndez-Menéndez, Y.; Otero-Hernández, J. CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture. World J. Stem Cells 2015, 7, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Buhring, H.J.; Treml, S.; Cerabona, F.; de Zwart, P.; Kanz, L.; Sobiesiak, M. Phenotypic characterization of distinct human bone marrow-derived MSC subsets. Ann. N. Y. Acad. Sci. 2009, 1176, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Quirici, N.; Soligo, D.; Bossolasco, P.; Servida, F.; Lumini, C.; Deliliers, G.L. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp. Hematol. 2002, 30, 783–791. [Google Scholar] [CrossRef]
- Kuci, S.; Kuci, Z.; Kreyenberg, H.; Deak, E.; Putsch, K.; Huenecke, S.; Amara, C.; Koller, S.; Rettinger, E.; Grez, M.; et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. Haematologica 2010, 95, 651–659. [Google Scholar] [CrossRef]
- Barilani, M.; Banfi, F.; Sironi, S.; Ragni, E.; Guillaumin, S.; Polveraccio, F.; Rosso, L.; Moro, M.; Astori, G.; Pozzobon, M.; et al. Low-affinity Nerve Growth Factor Receptor (CD271) Heterogeneous Expression in Adult and Fetal Mesenchymal Stromal Cells. Sci. Rep. 2018, 8, 9321. [Google Scholar] [CrossRef] [Green Version]
- Poblet, E.; Jiménez, F. CD10 and CD34 in fetal and adult human hair follicles: Dynamic changes in their immunohistochemical expression during embryogenesis and hair cycling. Br. J. Dermatol. 2008, 159, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Poblet, E.; Jiménez, F. CD34 in Human Hair Follicle. J. Investig. Dermatol. 2003, 121, 1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]



| Antibody | Immunoglobulin, Clone | Fluorochrome | Manufacturer |
|---|---|---|---|
| Anti-Human LGR6 | rabbit IgG | Unconjugated | Sigma-Aldrich GmbH, Steinheim, DE |
| Anti-Human Nestin | mIgG1, clone 10C2 | Unconjugated | ThermoFisher Scientific Inc., Waltham, USA |
| Anti-Human NGFR (CD271) | mIgG1, Clone ME20.4 | Unconjugated | ThermoFisher Scientific Inc., Waltham, USA |
| Anti-Human CK15 | mIgG1, Clone LHK15 | Unconjugated | Abcam Plc, Cambridge, USA |
| Anti-Human PAX3 | rabbit IgG | Unconjugated | ThermoFisher Scientific Inc., Waltham, USA |
| Anti-Human MITF | mIgG1κ, Clone D5 | Unconjugated | ThermoFisher Scientific Inc., Waltham, USA |
| Anti-human soluble adenylyl cyclase (sAC) | mlgG1, ADCY10 | Unconjugated | CEP Biotech Inc., Tamarac, USA |
| Anti-human Nestin | mIgG1, 10C2 | Unconjugated | Cell Signaling Technology, Inc., Danvers, USA |
| Anti-Human CD44 | mIgG2a, 156-3C11 | Unconjugated | Cell Signaling Technology, Inc., Danvers, USA |
| Anti-Human CD34 | mIgG1κ, B-6 | Unconjugated | Santa Cruz Biotechnology, Inc., Dallas, USA |
| Anti-Human CD133 | Rabbit IgG, D2V8Q | Unconjugated | Cell Signaling Technology, Inc., Danvers, USA |
| Anti-Human CD44 | mIgG2b, Clone G44-26 | PE | BD Biosciences, San Jose, USA |
| Anti-Human CD73 | mIgG1, Clone AD2 | APC | BD Biosciences, San Jose, USA |
| Anti-Human CD90 (Thy-1) | mIgG1, Clone 5E10 | FITC | BD Biosciences, San Jose, USA |
| Anti-Human CD105 (ENG) | mIgG1, Clone 266 | PerCP-Cy5.5 | BD Biosciences, San Jose, USA |
| MSC Negative Mixture | CD34, CD11b, CD19 PE, CD45 PE, HLA-DR | PE | BD Biosciences, San Jose, USA |
| Gene | Primer Sequence | |
|---|---|---|
| CK15 | For | AGTGGATGGACAGGTGGTTT |
| CK15 | Rev | CTGATGAGAGTGGGGAGTGG |
| CK5 | For | GCTGACACGAGAACCCAAAG |
| CK5 | Rev | ATTGGGGTGGGGATTCTGTT |
| CK10 | For | GGTGGTGGATTTGGAGGAGA |
| CK10 | Rev | TCTTCCAGAGCCCGAACTTT |
| TYR | For | AGTAATGTCCAGGTTCCCAGA |
| TYR | Rev | ATGGGCTTAGGGGAAAATGTT |
| PAX3 | For | CTGCGTCTCCAAGATCCTGT |
| PAX3 | Rev | TTTTCTTCTCCACGTCAGGC |
| MITF | For | CAGTGGTTTGGGCTTGTTGT |
| MITF | Rev | TGACCAGGTTGCTTGTATGC |
| MYC | For | ATTCTCTGCTCTCCTCGACG |
| MYC | Rev | AGCCTGCCTCTTTTCCACA |
| NES | For | CTGCGGGCTACTGAAAAGT |
| NES | Rev | GTTTGCAGCCGGGAGTTC |
| LGR6 | For | CAGGTGGAGGCTTGTCAGG |
| LGR6 | Rev | TCACACTGCTGAGTTTTGGT |
| NANOG | For | CCTATGCCTGTGATTTGTGGG |
| NANOG | Rev | AGTGGGTTGTTTGCCTTTGG |
| OCT4 | For | GGAGTTTGTGCCAGGGTTTT |
| OCT4 | Rev | TGTGTCCCAGGCTTCTTTATT |
| PMEL | For | ACTCTTTGACTCCTCACACAGC |
| PMEL | Rev | ATTTCAAATGGGGATCATAATGT |
| CKIT | For | ATCAGCGCATAACAGCCTAAT |
| CKIT | Rev | CCAGCAAAATCAGAGTTAATCG |
| CD73 | For | CTTTCGCACCCAGTTCACG |
| CD73 | Rev | TCGTTGGTGTGCAAAATCGT |
| CD45 | For | CTTAGGGACACGGCTGACTT |
| CD45 | Rev | TGCCCTGTCACAAATACTTCTG |
| NGFR | For | GGACGCCTCGGATTCTGTAG |
| NGFR | Rev | CTTCCAGGGCATTCGGTTCA |
| CD34 | For | CTACAACACCTAGTACCCTTGGA |
| CD34 | Rev | GGTGAACACTGTGCTGATTACA |
| HPRT1 | For | GCTTCCTCCTCCTCTGCC |
| HPRT1 | Rev | CACTAATCACAACGCTGGGG |
| CD44 | QT00073549, QuantiTect Primer Assays, Qiagen, DE | |
| CD133 | QT00075586, QuantiTect Primer Assays, Qiagen, DE | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Masieri, F.F.; Schneider, M.; Bartella, A.; Gaus, S.; Hahnel, S.; Zimmerer, R.; Sack, U.; Maksimovic-Ivanic, D.; Mijatovic, S.; et al. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules 2021, 11, 154. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020154
Li H, Masieri FF, Schneider M, Bartella A, Gaus S, Hahnel S, Zimmerer R, Sack U, Maksimovic-Ivanic D, Mijatovic S, et al. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules. 2021; 11(2):154. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020154
Chicago/Turabian StyleLi, Hanluo, Federica Francesca Masieri, Marie Schneider, Alexander Bartella, Sebastian Gaus, Sebastian Hahnel, Rüdiger Zimmerer, Ulrich Sack, Danijela Maksimovic-Ivanic, Sanja Mijatovic, and et al. 2021. "The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers" Biomolecules 11, no. 2: 154. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020154