Psychosocial Crowding Stress-Induced Changes in Synaptic Transmission and Glutamate Receptor Expression in the Rat Frontal Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Stress Procedures
2.3. Tissue Processing
2.4. ELISA Analysis of CORT, ACTH, and IL1B Levels
2.5. Morphometric Analysis of the Adrenal Glands
2.6. Field Potential, Paired-Pulse Stimulation Recordings, Long-Term Potentiation (LTP) Induction, and Data Analysis
2.7. Immunoblotting Data Generation and Analysis
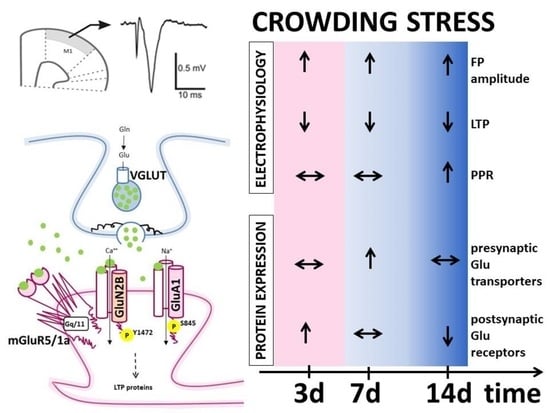
3. Results
3.1. Rat Body Response to Crowding Stress Exposure
3.1.1. Body Weight
3.1.2. Plasma Stress Hormone Levels
3.1.3. Adrenal Morphometry, Thymus, and Spleen Weight after 3 Days of Crowding Stress
3.2. The Effects of Different Durations of Crowding Stress on Field Potentials
3.3. The Effects of Different Time Exposure to Crowding Stress on Paired-Pulse Ratio
3.4. The Effects of Different Durations of Crowding Stress on Long-Term Potentiation
3.5. The Effects of Different Durations of Crowding Stress on GluA1, GluN2B, and mGluR5/1a Expression in the Rat FC
3.5.1. GluA1 Protein
3.5.2. GluN2B Protein
3.5.3. mGluR5/1a Protein Dimer
3.6. The Effects of Different Durations of Crowding Stress on the Level of Vesicular Glutamate Transporters VGLUT1 and VGLUT2 in the Rat FC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSF | artificial cerebrospinal fluid |
| ACTH | adrenocorticotropic hormone |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| CORT | corticosterone |
| CS | animal model of psychosocial crowding stress |
| ELISA | enzyme-linked immunosorbent assay |
| FC | frontal cortex |
| FP | field potentials |
| GR | glucocorticoid receptor |
| HPA | hypothalamic-pituitary-adrenal axis |
| Il 1β | interleukin 1 beta |
| LTP | long term potentiation |
| NMDA | N-methyl-d-aspartate |
| PPR | Paired-pulse ratio |
References
- Blanchard, R.J.; McKittrick, C.R.; Blanchard, D.C. Animal models of social stress: Effects on behavior and brain neurochemical systems. Physiol. Behav. 2001, 73, 261–271. [Google Scholar] [CrossRef]
- Kusek, M.; Tokarska, A.; Siwiec, M.; Gadek-Michalska, A.; Szewczyk, B.; Hess, G.; Tokarski, K. Nitric Oxide Synthase Inhibitor Attenuates the Effects of Repeated Restraint Stress on Synaptic Transmission in the Paraventricular Nucleus of the Rat Hypothalamus. Front. Cell. Neurosci. 2017, 11, 127. [Google Scholar] [CrossRef] [Green Version]
- Martí, O.; Armario, A. Anterior pituitary response to stress: Time-related changes and adaptation. Int. J. Dev. Neurosci. 1998, 16, 241–260. [Google Scholar] [CrossRef]
- Girotti, M.; Pace, T.; Gaylord, R.; Rubin, B.; Herman, J.; Spencer, R. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience 2006, 138, 1067–1081. [Google Scholar] [CrossRef]
- Shields, G.S.; Sazma, M.A.; Yonelinas, A.P. The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neurosci. Biobehav. Rev. 2016, 68, 651–668. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.R.; Myers, B.; Herman, J.P. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol. Behav. 2011, 104, 266–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep Brain Stimulation for Treatment-Resistant Depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Wood, J.; Bondi, C.; Del Arco, A.; Moghaddam, B. Anxiety Evokes Hypofrontality and Disrupts Rule-Relevant Encoding by Dorsomedial Prefrontal Cortex Neurons. J. Neurosci. 2016, 36, 3322–3335. [Google Scholar] [CrossRef] [Green Version]
- McGinty, J.F.; Zelek-Molik, A.; Sun, W.-L. Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal. Brain Res. 2015, 1628, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamp, D.; Brinkmeyer, J.; Agelink, M.W.; Habakuck, M.; Mobascher, A.; Wölwer, W.; Cordes, J. High frequency repetitive transcranial magnetic stimulation (rTMS) reduces EEG-hypofrontality in patients with schizophrenia. Psychiatry Res. 2016, 236, 199–201. [Google Scholar] [CrossRef]
- Musazzi, L.; Treccani, G.; Popoli, M. Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front. Psychiatry 2015, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Shi, Y.; Jackson, A.C.; Bjorgan, K.; During, M.J.; Sprengel, R.; Seeburg, P.H.; Nicoll, R.A. Subunit Composition of Synaptic AMPA Receptors Revealed by a Single-Cell Genetic Approach. Neuron 2009, 62, 254–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, T.; Moriyoshi, K.; Sugihara, H.; Sakurada, K.; Kadotani, H.; Yokoi, M.; Akazawa, C.; Shigemoto, R.; Mizuno, N.; Masu, M. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J. Biol. Chem. 1993, 268, 2836–2843. [Google Scholar] [CrossRef]
- Wyllie, D.; Livesey, M.; Hardingham, G. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology 2013, 74, 4–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikics, E.; Toth, M.; Biro, L.; Bruzsik, B.; Nagy, B.; Haller, J. The role of GluN2B-containing NMDA receptors in short- and long-term fear recall. Physiol. Behav. 2017, 177, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kiselycznyk, C.; Svenningsson, P.; Delpire, E.; Holmes, A. Genetic, pharmacological and lesion analyses reveal a selective role for corticohippocampal GLUN2B in a novel repeated swim stress paradigm. Neuroscience 2011, 193, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Chaki, S.; Ago, Y.; Palucha-Paniewiera, A.; Matrisciano, F.; Pilc, A. mGlu2/3 and mGlu5 receptors: Potential targets for novel antidepressants. Neuropharmacology 2013, 66, 40–52. [Google Scholar] [CrossRef]
- Pin, J.P.; Galvez, T.; Prézeau, L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003, 98, 325–354. [Google Scholar] [CrossRef]
- Jones, O.D. Do group I metabotropic glutamate receptors mediate LTD? Neurobiol. Learn. Mem. 2017, 138, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Bellone, C.; Lüscher, C.; Mameli, M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell. Mol. Life Sci. 2008, 65, 2913–2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugajski, J.; Gadek-Michalska, A.; Bugajski, A.J. Effect of cyclooxygenase inhibitors on the CHR-induced pituitary-adrenocortical activity during crowding stress. J. Physiol. Pharmacol. 2003, 54, 99–108. [Google Scholar] [PubMed]
- Brydon, L.; Edwards, S.; Jia, H.; Mohamed-Ali, V.; Zachary, I.; Martin, J.F.; Steptoe, A. Psychological stress activates interleukin-1β gene expression in human mononuclear cells. Brain. Behav. Immun. 2005, 19, 540–546. [Google Scholar] [CrossRef]
- Zou, W.; Feng, R.; Yang, Y. Changes in the serum levels of inflammatory cytokines in antidepressant drug-naïve patients with major depression. PLoS ONE 2018, 13, e0197267. [Google Scholar] [CrossRef]
- Bobula, B.; Sowa, J.; Hess, G. Anti-interleukin-1β antibody prevents the occurrence of repeated restraint stress-induced alterations in synaptic transmission and long-term potentiation in the rat frontal cortex. Pharmacol. Rep. 2015, 67, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.J.; Grunberg, N.E. Effects of housing on male and female rats: Crowding stresses males but calms females. Physiol. Behav. 1995, 58, 1085–1089. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Psychosocial stress inhibits additional stress-induced hyperexpression of NO synthases and IL-1β in brain structures. Pharmacol. Rep. 2016, 68, 1178–1196. [Google Scholar] [PubMed]
- Chmielarz, P.; Kreiner, G.; Nalepa, I. Selective ablation of glucocorticoid receptors in the noradrenergic system affects evening corticosterone levels in a sex-dependent manner. Pharmacol. Rep. 2015, 67, 1201–1203. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Kreiner, G.; Kuśmierczyk, J.; Kowalska, M.; Roman, A.; Tota, K.; Nalepa, I. Depressive-like immobility behavior and genotype× stress interactions in male mice of selected strains. Stress 2016, 19, 206–213. [Google Scholar] [CrossRef]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Spyrka, J.; Bugajski, J. Effect of prior stress on interleukin-1β and HPA axis responses to acute stress. Pharmacol. Rep. 2011, 63, 1393–1403. [Google Scholar]
- Schwendt, M.; Rocha, A.; See, R.E.; Pacchioni, A.M.; McGinty, J.F.; Kalivas, P.W. Extended Methamphetamine Self-Administration in Rats Results in a Selective Reduction of Dopamine Transporter Levels in the Prefrontal Cortex and Dorsal Striatum Not Accompanied by Marked Monoaminergic Depletion. J. Pharmacol. Exp. Ther. 2009, 331, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Zelek-Molik, A.; McGinty, J.F. Short and long access to cocaine self-administration activates tyrosine phosphatase STEP and attenuates GluN expression but differentially regulates GluA expression in the prefrontal cortex. Psychopharmacology 2013, 229, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Tamashiro, K.L.K.; Hegeman, M.A.; Nguyen, M.M.N.; Melhorn, S.J.; Ma, L.Y.; Woods, S.C.; Sakai, R.R. Dynamic body weight and body composition changes in response to subordination stress. Physiol. Behav. 2007, 91, 440–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gądek-Michalska, A.; Spyrka, J.; Rachwalska, P.; Tadeusz, J.; Bugajski, J. Influence of chronic stress on brain corticosteroid receptors and HPA axis activity. Pharmacol. Rep. 2013, 65, 1163–1175. [Google Scholar] [CrossRef]
- Viau, V.; Meaney, M. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J. Neurosci. 1996, 16, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- Bugajski, J.; Gądek-Michalska, A.; Bugajski, A.J. Nitric Oxide and Prostaglandin Systems in the. J. Physiol. Pharmacol. 2004, 55, 679–703. [Google Scholar] [PubMed]
- Porterfield, V.; Zimomra, Z.; Caldwell, E.; Camp, R.; Gabella, K.; Johnson, J. Rat strain differences in restraint stress-induced brain cytokines. Neuroscience 2011, 188, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Barnard, D.F.; Gabella, K.M.; Kulp, A.C.; Parker, A.D.; Dugan, P.B.; Johnson, J.D. Sex differences in the regulation of brain IL-1β in response to chronic stress. Psychoneuroendocrinology 2019, 103, 203–211. [Google Scholar] [CrossRef]
- Bobula, B.; Wabno, J.; Hess, G. Imipramine counteracts corticosterone-induced enhancement of glutamatergic transmission and impairment of long-term potentiation in the rat frontal cortex. Pharmacol. Rep. 2011, 63, 1404–1412. [Google Scholar]
- Sowa, J.; Bobula, B.; Glombik, K.; Slusarczyk, J.; Basta-Kaim, A.; Hess, G. Prenatal Stress Enhances Excitatory Synaptic Transmission and Impairs Long-Term Potentiation in the Frontal Cortex of Adult Offspring Rats. PLoS ONE 2015, 10, e0119407. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, B. Stress Preferentially Increases Extraneuronal Levels of Excitatory Amino Acids in the Prefrontal Cortex: Comparison to Hippocampus and Basal Ganglia. J. Neurochem. 1993, 60, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, D.; Tendilla-Beltrán, H.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C.; Caso, J.R. Chronic Mild Stress Alters Kynurenine Pathways Changing the Glutamate Neurotransmission in Frontal Cortex of Rats. Mol. Neurobiol. 2019, 56, 490–501. [Google Scholar] [CrossRef]
- Zarate, C.; Manji, H.K. Riluzole in psychiatry: A systematic review of the literature. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.T.; Bains, S.; Jeetle, J.; Whelpton, R. Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex--a possible mechanism of neuroprotection in major depression? Neuroscience 2000, 100, 681–684. [Google Scholar] [CrossRef]
- Gołembiowska, K.; Dziubina, A. Effect of acute and chronic administration of citalopram on glutamate and aspartate release in the rat prefrontal cortex. Pol. J. Pharmacol. 2001, 52, 441–448. [Google Scholar]
- Katz, B.; Miledi, R. The role of calcium in neuromuscular facilitation. J. Physiol. 1968, 195, 481–492. [Google Scholar] [CrossRef]
- Hess, G.; Aizenman, C.D.; Donoghue, J.P. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J. Neurophysiol. 1996, 75, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.J.; Wang, Q.; Zheng, X.X.; Cheng, Y.; Zhang, Y. Involvement of SNARE complex in the hippocampus and prefrontal cortex of offspring with depression induced by prenatal stress. J. Affect. Disord. 2018, 235, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Spyrka, J.; Hess, G. Repeated restraint-induced modulation of long-term potentiation in the dentate gyrus of the mouse. Brain Res. 2010, 1320, 28–33. [Google Scholar] [CrossRef]
- Danielewicz, J.; Hess, G. Early life stress alters synaptic modification range in the rat lateral amygdala. Behav. Brain Res. 2014, 265, 32–37. [Google Scholar] [CrossRef]
- Sripanidkulchai, K.; Sripanidkulchai, B.; Wyss, J.M. The cortical projection of the basolateral amygdaloid nucleus in the rat: A retrograde fluorescent dye study. J. Comp. Neurol. 1984, 229, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Li, A.; Zheng, Y.; Han, Y.; Tian, J.; Xu, Z.; Gong, H.; Li, X. Whole Brain Mapping of Long-Range Direct Input to Glutamatergic and GABAergic Neurons in Motor Cortex. Front. Neuroanat. 2019, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Chocyk, A.; Bobula, B.; Dudys, D.; Przyborowska, A.; Majcher-Maślanka, I.; Hess, G.; Wędzony, K. Early-life stress affects the structural and functional plasticity of the medial prefrontal cortex in adolescent rats. Eur. J. Neurosci. 2013, 38, 2089–2107. [Google Scholar] [CrossRef]
- Dupin, N.; Mailliet, F.; Rocher, C.; Kessal, K.; Spedding, M.; Jay, T.M. Common efficacy of psychotropic drugs in restoring stress-induced impairment of prefrontal plasticity. Neurotox. Res. 2006, 10, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Yuen, E.Y.; Liu, W.; Karatsoreos, I.N.; Feng, J.; McEwen, B.S.; Yan, Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc. Natl. Acad. Sci. USA 2009, 106, 14075–14079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horak, M.; Petralia, R.S.; Kaniakova, M.; Sans, N. ER to synapse trafficking of NMDA receptors. Front. Cell. Neurosci. 2014, 8, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goebel-Goody, S.M.; Baum, M.; Paspalas, C.D.; Fernandez, S.M.; Carty, N.C.; Kurup, P.; Lombroso, P.J. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 65–87. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Wei, J.; Liu, W.; Zhong, P.; Li, X.; Yan, Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 2012, 73, 962–977. [Google Scholar] [CrossRef] [Green Version]
- Liguz-Lecznar, M.; Skangiel-Kramska, J. Vesicular glutamate transporters (VGLUTs): The three musketeers of glutamatergic system. Acta Neurobiol. Exp. 2007, 67, 207–218. [Google Scholar]
- Gorelova, N.; Mulholland, P.J.; Chandler, L.J.; Seamans, J.K. The Glutamatergic Component of the Mesocortical Pathway Emanating from Different Subregions of the Ventral Midbrain. Cereb. Cortex 2011, 22, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Bunse, T.; Wobrock, T.; Strube, W.; Padberg, F.; Palm, U.; Falkai, P.; Hasan, A. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: A systematic review. Brain. Stimul. 2014, 7, 158–169. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelek-Molik, A.; Bobula, B.; Gądek-Michalska, A.; Chorązka, K.; Bielawski, A.; Kuśmierczyk, J.; Siwiec, M.; Wilczkowski, M.; Hess, G.; Nalepa, I. Psychosocial Crowding Stress-Induced Changes in Synaptic Transmission and Glutamate Receptor Expression in the Rat Frontal Cortex. Biomolecules 2021, 11, 294. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020294
Zelek-Molik A, Bobula B, Gądek-Michalska A, Chorązka K, Bielawski A, Kuśmierczyk J, Siwiec M, Wilczkowski M, Hess G, Nalepa I. Psychosocial Crowding Stress-Induced Changes in Synaptic Transmission and Glutamate Receptor Expression in the Rat Frontal Cortex. Biomolecules. 2021; 11(2):294. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020294
Chicago/Turabian StyleZelek-Molik, Agnieszka, Bartosz Bobula, Anna Gądek-Michalska, Katarzyna Chorązka, Adam Bielawski, Justyna Kuśmierczyk, Marcin Siwiec, Michał Wilczkowski, Grzegorz Hess, and Irena Nalepa. 2021. "Psychosocial Crowding Stress-Induced Changes in Synaptic Transmission and Glutamate Receptor Expression in the Rat Frontal Cortex" Biomolecules 11, no. 2: 294. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11020294