Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?—An Overview
Abstract
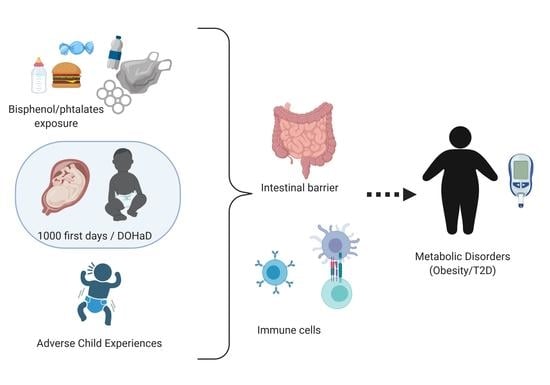
:1. Introduction
2. Early Origins of Metabolic Disorders
3. Food Contaminants or Social Stress Exposure in Early Life Are Suspected to Contribute to Metabolic Disorders
3.1. Role of Environmental Contaminants
3.1.1. Focus on Endocrine Disruptors Chemicals (EDCs): Bisphenols and Phthalates
3.1.2. EDCs Could Trigger MD in Offspring by Inducing Gestational Diabetes Mellitus (GDM)
3.2. Role of Social Stress Exposure in Early Life and MD
4. Evidence for a Role of ELAE on MD Occurrence in Animal Models
4.1. Link between Environmental Contaminants and Weight Gain
4.2. Link between Environmental Contaminants and T2D
4.3. Link between Social Stress and Metabolic Disorders
5. Hypothesis of Different Mechanisms Leading to MD
5.1. Actors Involved in MD
5.1.1. Intestinal Barrier—Contribution in Immune Response Commitment
5.1.2. Immune System—Chronic low-Grade Inflammation
5.2. Role of Intestinal Barrier (IB) and Inflammation in Early Life Exposure to EDC-Induced MD
5.3. Role of Intestinal Barrier and Inflammation in Early Life Stress-Induced MD
5.4. Role of Epigenetics in Early Imprinting
6. Conclusions and Perspective on Enriched Environments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Adverse Childhood Experiences |
| BMI | Body Mass Index |
| BP | Bisphenol |
| CRP | C Reactive Protein |
| DEHP | di (2-ethylhexyl) phthalate |
| DEP | diethyl phthalate |
| DOHaD | Developmental Origin of Health and Diseases |
| EDC | Endocrine disrupting chemicals |
| ELAE | Early Life Adverse Events |
| GDM | Gestational Diabetes Mellitus |
| HFD | High Fat Diet |
| IB | Intestinal Barrier |
| IBS | Irritable Bowel Syndrome |
| IFG | Impaired Fasting Glycemia |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IGF | Impaired Glucose Tolerance |
| IL | Interleukin |
| ILC | Innate lymphoid cells |
| LBW | Low Birth Weight |
| MD | Metabolic Disorders |
| MDC | Metabolism Disrupting Chemical |
| MEP | Mono-ethyl-phtalate |
| MPO | Myeloperoxidase |
| MS | Maternal Separation |
| PND | Post Natal Day |
| SEP | Socio Economic Position |
| T2D | Type 2 Diabetes |
| Th | T helper cells |
| Treg | Regulatory T cells |
| WAT | White Adipose Tissue |
References
- Clark, H.; Coll-Seck, A.M.; Banerjee, A.; Peterson, S.; Dalglish, S.L.; Ameratunga, S.; Balabanova, D.; Bhan, M.K.; Bhutta, Z.A.; Borrazzo, J.; et al. A Future for the World’s Children? A WHO–UNICEF–Lancet Commission. Lancet 2020, 395, 605–658. [Google Scholar] [CrossRef] [Green Version]
- Gluckman, P.D.; Buklijas, T.; Hanson, M.A. The Developmental Origins of Health and Disease (DOHaD) Concept. In The Epigenome and Developmental Origins of Health and Disease; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–15. ISBN 978-0-12-801383-0. [Google Scholar]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The Microbiome in Early Life: Implications for Health Outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Fulde, M.; Hornef, M.W. Maturation of the Enteric Mucosal Innate Immune System during the Postnatal Period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal Exposure to the Dutch Famine and Disease in Later Life: An Overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Ren, W.; Luo, R.; Zhang, S.; Zhang, J.H.; Zeng, Q. Risk of Metabolic Syndrome in Adults Exposed to the Great Chinese Famine during the Fetal Life and Early Childhood. Eur. J. Clin. Nutr. 2012, 66, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hult, M.; Tornhammar, P.; Ueda, P.; Chima, C.; Bonamy, A.-K.E.; Ozumba, B.; Norman, M. Hypertension, Diabetes and Overweight: Looming Legacies of the Biafran Famine. PLoS ONE 2010, 5, e13582. [Google Scholar] [CrossRef] [Green Version]
- No name Practice Bulletin No 156: Obesity in Pregnancy. Obstet. Gynecol. 2015, 126, e112–e126. [CrossRef] [PubMed]
- Solveig A Cunningham, Michael R Kramer, K M Venkat Narayan Incidence of Childhood Obesity in the United States. N. Engl. J. Med. 2014, 370, 1659–1661. [CrossRef] [Green Version]
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; de Mouzon, S.H.; Amini, S.B. Perinatal Risk Factors for Childhood Obesity and Metabolic Dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, R.; Rurangirwa, A.A.; Williams, M.A.; Hofman, A.; Mackenbach, J.P.; Franco, O.H.; Steegers, E.A.P.; Jaddoe, V.W.V. Maternal Parity, Fetal and Childhood Growth, and Cardiometabolic Risk Factors. Hypertension 2014, 64, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Kaar, J.L.; Crume, T.; Brinton, J.T.; Bischoff, K.J.; McDuffie, R.; Dabelea, D. Maternal Obesity, Gestational Weight Gain, and Offspring Adiposity: The Exploring Perinatal Outcomes among Children Study. J. Pediatrics 2014, 165, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.; Steegers, E.A.P.; Duijts, L.; Felix, J.F.; Hofman, A.; Franco, O.H.; Jaddoe, V.W.V. Childhood Cardiometabolic Outcomes of Maternal Obesity During Pregnancy: The Generation R Study. Hypertension 2014, 63, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karachaliou, M.; Georgiou, V.; Roumeliotaki, T.; Chalkiadaki, G.; Daraki, V.; Koinaki, S.; Dermitzaki, E.; Sarri, K.; Vassilaki, M.; Kogevinas, M.; et al. Association of Trimester-Specific Gestational Weight Gain with Fetal Growth, Offspring Obesity, and Cardiometabolic Traits in Early Childhood. Am. J. Obstet. Gynecol. 2015, 212, 502.e1–502.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gingras, V.; Hivert, M.-F.; Oken, E. Early-Life Exposures and Risk of Diabetes Mellitus and Obesity. Curr. Diab. Rep. 2018, 18, 89. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.P. Type 2 (Non-Insulin-Dependent) Diabetes Mellitus: The Thrifty Phenotype Hypothesis. Diabetologia 1992, 35, 595–601. [Google Scholar] [CrossRef]
- Rughani, A.; Friedman, J.E.; Tryggestad, J.B. Type 2 Diabetes in Youth: The Role of Early Life Exposures. Curr. Diab. Rep. 2020, 20, 45. [Google Scholar] [CrossRef]
- Chevalier, N.; Fénichel, P. Endocrine Disruptors: New Players in the Pathophysiology of Type 2 Diabetes? Diabetes Metab. 2015, 41, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism Disrupting Chemicals and Metabolic Disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [Green Version]
- Li, L.-X.; Chen, L.; Meng, X.-Z.; Chen, B.-H.; Chen, S.-Q.; Zhao, Y.; Zhao, L.-F.; Liang, Y.; Zhang, Y.-H. Exposure Levels of Environmental Endocrine Disruptors in Mother-Newborn Pairs in China and Their Placental Transfer Characteristics. PLoS ONE 2013, 8, e62526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heindel, J.J.; Balbus, J.; Birnbaum, L.; Brune-Drisse, M.N.; Grandjean, P.; Gray, K.; Landrigan, P.J.; Sly, P.D.; Suk, W.; Cory Slechta, D.; et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology 2015, 156, 3416–3421. [Google Scholar] [CrossRef] [Green Version]
- Malaisé, Y.; Lencina, C.; Cartier, C.; Olier, M.; Ménard, S.; Guzylack-Piriou, L. Perinatal Oral Exposure to Low Doses of Bisphenol A, S or F Impairs Immune Functions at Intestinal and Systemic Levels in Female Offspring Mice. Environ. Health 2020, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Toner, F.; Allan, G.; Dimond, S.S.; Waechter, J.M.; Beyer, D. In Vitro Percutaneous Absorption and Metabolism of Bisphenol A (BPA) through Fresh Human Skin. Toxicol In Vitro 2018, 47, 147–155. [Google Scholar] [CrossRef]
- Strakovsky, R.S.; Schantz, S.L. Using Experimental Models to Assess Effects of Bisphenol A (BPA) and Phthalates on the Placenta: Challenges and Perspectives. Toxicol. Sci. 2018, 166, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Grandin, F.C.; Lacroix, M.Z.; Gayrard, V.; Viguié, C.; Mila, H.; de Place, A.; Vayssière, C.; Morin, M.; Corbett, J.; Gayrard, C.; et al. Is Bisphenol S a Safer Alternative to Bisphenol A in Terms of Potential Fetal Exposure? Placental Transfer across the Perfused Human Placenta. Chemosphere 2019, 221, 471–478. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Zhang, D.; Chen, Z. In Vivo Toxicity Assessment of Deoxynivalenol-Contaminated Wheat after Ozone Degradation. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2016. [Google Scholar] [CrossRef]
- Almeida, D.L.; Pavanello, A.; Saavedra, L.P.; Pereira, T.S.; de Castro-Prado, M.A.A.; de Freitas Mathias, P.C. Environmental Monitoring and the Developmental Origins of Health and Disease. J. Dev. Orig Health Dis. 2019, 10, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Lanphear, B.P.; Calafat, A.M.; Deria, S.; Khoury, J.; Howe, C.J.; Venners, S.A. Early-Life Bisphenol a Exposure and Child Body Mass Index: A Prospective Cohort Study. Environ. Health Perspect. 2014, 122, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Lang, I.A. Association of Urinary Bisphenol A Concentration With Medical Disorders and Laboratory Abnormalities in Adults. JAMA 2008, 300, 1303. [Google Scholar] [CrossRef]
- Rancière, F.; Lyons, J.G.; Loh, V.H.Y.; Botton, J.; Galloway, T.; Wang, T.; Shaw, J.E.; Magliano, D.J. Bisphenol A and the Risk of Cardiometabolic Disorders: A Systematic Review with Meta-Analysis of the Epidemiological Evidence. Environ. Health 2015, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Harley, K.G.; Aguilar Schall, R.; Chevrier, J.; Tyler, K.; Aguirre, H.; Bradman, A.; Holland, N.T.; Lustig, R.H.; Calafat, A.M.; Eskenazi, B. Prenatal and Postnatal Bisphenol A Exposure and Body Mass Index in Childhood in the CHAMACOS Cohort. Environ. Health Perspect. 2013, 121, 514–520. [Google Scholar] [CrossRef]
- Valvi, D.; Oulhote, Y.; Weihe, P.; Dalgård, C.; Bjerve, K.S.; Steuerwald, U.; Grandjean, P. Gestational Diabetes and Offspring Birth Size at Elevated Environmental Pollutant Exposures. Environ. Int. 2017, 107, 205–215. [Google Scholar] [CrossRef]
- Agay-Shay, K.; Martinez, D.; Valvi, D.; Garcia-Esteban, R.; Basagaña, X.; Robinson, O.; Casas, M.; Sunyer, J.; Vrijheid, M. Exposure to Endocrine-Disrupting Chemicals during Pregnancy and Weight at 7 Years of Age: A Multi-Pollutant Approach. Environ. Health Perspect. 2015, 123, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bovet, P.; Ma, C.; Zhao, M.; Liang, Y.; Xi, B. Prevalence of Underweight and Overweight among Young Adolescents Aged 12-15 Years in 58 Low-Income and Middle-Income Countries. Pediatr. Obes. 2019, 14, e12468. [Google Scholar] [CrossRef] [PubMed]
- Freinkel, N. Gestational Diabetes 1979: Philosophical and Practical Aspects of a Major Public Health Problem. Diabetes Care 1980, 3, 399–401. [Google Scholar] [CrossRef] [PubMed]
- DeSisto, C.L.; Kim, S.Y.; Sharma, A.J. Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev. Chronic Dis. 2014, 11, E104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [Green Version]
- Delpierre, C.; Fantin, R.; Barboza-Solis, C.; Lepage, B.; Darnaudéry, M.; Kelly-Irving, M. The Early Life Nutritional Environment and Early Life Stress as Potential Pathways towards the Metabolic Syndrome in Mid-Life? A Lifecourse Analysis Using the 1958 British Birth Cohort. BMC Public Health 2016, 16, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philips, E.M.; Santos, S.; Steegers, E.A.P.; Asimakopoulos, A.G.; Kannan, K.; Trasande, L.; Jaddoe, V.W.V. Maternal Bisphenol and Phthalate Urine Concentrations and Weight Gain during Pregnancy. Environ. Int. 2020, 135, 105342. [Google Scholar] [CrossRef]
- Bellavia, A.; Hauser, R.; Seely, E.W.; Meeker, J.D.; Ferguson, K.K.; McElrath, T.F.; James-Todd, T. Urinary Phthalate Metabolite Concentrations and Maternal Weight during Early Pregnancy. Int. J. Hyg. Environ. Health 2017, 220, 1347–1355. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Chen, Q.; Luo, Z.-C.; Zhao, S.; Wang, W.; Zhang, H.-J.; Zhang, J.; Ouyang, F. Urinary Bisphenol A Concentration and Gestational Diabetes Mellitus in Chinese Women. Epidemiology 2017, 28 (Suppl. 1), S41–S47. [Google Scholar] [CrossRef]
- Robledo, C.; Peck, J.D.; Stoner, J.A.; Carabin, H.; Cowan, L.; Koch, H.M.; Goodman, J.R. Is Bisphenol-A Exposure during Pregnancy Associated with Blood Glucose Levels or Diagnosis of Gestational Diabetes? J. Toxicol Environ. Health A 2013, 76, 865–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, G.D.; Dodds, L.; Arbuckle, T.E.; Ashley-Martin, J.; Fraser, W.; Fisher, M.; Taback, S.; Keely, E.; Bouchard, M.F.; Monnier, P.; et al. Exposure to Phthalates, Bisphenol A and Metals in Pregnancy and the Association with Impaired Glucose Tolerance and Gestational Diabetes Mellitus: The MIREC Study. Environ. Int. 2015, 83, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Magdalena, P.; García-Arévalo, M.; Quesada, I.; Nadal, Á. Bisphenol-A Treatment during Pregnancy in Mice: A New Window of Susceptibility for the Development of Diabetes in Mothers Later in Life. Endocrinology 2015, 156, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James-Todd, T.M.; Meeker, J.D.; Huang, T.; Hauser, R.; Ferguson, K.K.; Rich-Edwards, J.W.; McElrath, T.F.; Seely, E.W. Pregnancy Urinary Phthalate Metabolite Concentrations and Gestational Diabetes Risk Factors. Environ. Int. 2016, 96, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, R.M.; Ferguson, K.K.; Sheppard, L.; James-Todd, T.; Butts, S.; Chandrasekaran, S.; Swan, S.H.; Barrett, E.S.; Nguyen, R.; Bush, N.; et al. Maternal Urinary Phthalate Metabolites in Relation to Gestational Diabetes and Glucose Intolerance during Pregnancy. Environ. Int. 2019, 123, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Robledo, C.A.; Peck, J.D.; Stoner, J.; Calafat, A.M.; Carabin, H.; Cowan, L.; Goodman, J.R. Urinary Phthalate Metabolite Concentrations and Blood Glucose Levels during Pregnancy. Int. J. Hyg. Environ. Health 2015, 218, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selye, H. A Syndrome Produced by Diverse Nocuous Agents. Nature 1936, 138, 32. [Google Scholar] [CrossRef]
- Danese, A.; McEwen, B.S. Adverse Childhood Experiences, Allostasis, Allostatic Load, and Age-Related Disease. Physiol. Behav. 2012, 106, 29–39. [Google Scholar] [CrossRef]
- Thomas, C.; Hyppönen, E.; Power, C. Obesity and Type 2 Diabetes Risk in Midadult Life: The Role of Childhood Adversity. Pediatrics 2008, 121, e1240–e1249. [Google Scholar] [CrossRef]
- Alastalo, H.; Raikkonen, K.; Pesonen, A.-K.; Osmond, C.; Barker, D.J.P.; Kajantie, E.; Heinonen, K.; Forsen, T.J.; Eriksson, J.G. Cardiovascular Health of Finnish War Evacuees 60 Years Later. Ann. Med. 2009, 41, 66–72. [Google Scholar] [CrossRef]
- Huang, H.; Yan, P.; Shan, Z.; Chen, S.; Li, M.; Luo, C.; Gao, H.; Hao, L.; Liu, L. Adverse Childhood Experiences and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Metabolism 2015, 64, 1408–1418. [Google Scholar] [CrossRef]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Davey Smith, G. Indicators of Socioeconomic Position (Part 1). J. Epidemiol. Community Health 2006, 60, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Cheval, B.; Chabert, C.; Orsholits, D.; Sieber, S.; Guessous, I.; Blane, D.; Kliegel, M.; Janssens, J.-P.; Burton-Jeangros, C.; Pison, C.; et al. Disadvantaged Early-Life Socioeconomic Circumstances Are Associated With Low Respiratory Function in Older Age. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1134–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, A.; Glymour, M.M.; Kawachi, I.; VanderWeele, T.J. Using Marginal Structural Models to Estimate the Direct Effect of Adverse Childhood Social Conditions on Onset of Heart Disease, Diabetes, and Stroke. Epidemiology 2012, 23, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cable, N. Life Course Approach in Social Epidemiology: An Overview, Application and Future Implications. J. Epidemiol. 2014, 24, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejlskov, L.; Bøggild, H.; Hansen, C.D.; Wulff, J.; Hansen, S.M.; Starkopf, L.; Lange, T.; Gerds, T.; Torp-Pedersen, C. The Effect of Early-Life and Adult Socioeconomic Position on Development of Lifestyle-Related Diseases. Eur. J. Public Health 2019, 29, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Rich-Edwards, J.W.; Spiegelman, D.; Lividoti Hibert, E.N.; Jun, H.-J.; Todd, T.J.; Kawachi, I.; Wright, R.J. Abuse in Childhood and Adolescence as a Predictor of Type 2 Diabetes in Adult Women. Am. J. Prev. Med. 2010, 39, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.E.; Lehman, B.J.; Kiefe, C.I.; Seeman, T.E. Relationship of Early Life Stress and Psychological Functioning to Adult C-Reactive Protein in the Coronary Artery Risk Development in Young Adults Study. Biol. Psychiatry 2006, 60, 819–824. [Google Scholar] [CrossRef]
- Boynton-Jarrett, R.; Rosenberg, L.; Palmer, J.R.; Boggs, D.A.; Wise, L.A. Child and Adolescent Abuse in Relation to Obesity in Adulthood: The Black Women’s Health Study. Pediatrics 2012, 130, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Bellis, M.A.; Hughes, K.; Leckenby, N.; Hardcastle, K.A.; Perkins, C.; Lowey, H. Measuring Mortality and the Burden of Adult Disease Associated with Adverse Childhood Experiences in England: A National Survey. J. Public Health 2015, 37, 445–454. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- García-Arévalo, M.; Alonso-Magdalena, P.; Servitja, J.-M.; Boronat-Belda, T.; Merino, B.; Villar-Pazos, S.; Medina-Gómez, G.; Novials, A.; Quesada, I.; Nadal, A. Maternal Exposure to Bisphenol-A During Pregnancy Increases Pancreatic β-Cell Growth During Early Life in Male Mice Offspring. Endocrinology 2016, 157, 4158–4171. [Google Scholar] [CrossRef] [PubMed]
- Malaisé, Y.; Menard, S.; Cartier, C.; Gaultier, E.; Lasserre, F.; Lencina, C.; Harkat, C.; Geoffre, N.; Lakhal, L.; Castan, I.; et al. Gut Dysbiosis and Impairment of Immune System Homeostasis in Perinatally-Exposed Mice to Bisphenol A Precede Obese Phenotype Development. Sci. Rep. 2017, 7, 14472. [Google Scholar] [CrossRef] [Green Version]
- Delclos, K.B.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Latendresse, J.R.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Gamboa da Costa, G.; Woodling, K.A.; et al. Toxicity Evaluation of Bisphenol A Administered by Gavage to Sprague Dawley Rats from Gestation Day 6 through Postnatal Day 90. Toxicol. Sci. 2014, 139, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Tyl, R.W.; Myers, C.B.; Marr, M.C.; Thomas, B.F.; Keimowitz, A.R.; Brine, D.R.; Veselica, M.M.; Fail, P.A.; Chang, T.Y.; Seely, J.C.; et al. Three-Generation Reproductive Toxicity Study of Dietary Bisphenol A in CD Sprague-Dawley Rats. Toxicol. Sci. 2002, 68, 121–146. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Quesada, I.; Nadal, A. Endocrine Disruptors in the Etiology of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2011, 7, 346–353. [Google Scholar] [CrossRef]
- Manikkam, M.; Tracey, R.; Guerrero-Bosagna, C.; Skinner, M.K. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Obesity, Reproductive Disease and Sperm Epimutations. PLoS ONE 2013, 8, e55387. [Google Scholar] [CrossRef]
- Schmidt, J.; Kotnik, P.; Trontelj, J.; Knez, Ž.; Mašič, L.P. Bioactivation of Bisphenol A and Its Analogs (BPF, BPAF, BPZ and DMBPA) in Human Liver Microsomes. Toxicol. In Vitro 2013, 27, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Angle, B.M.; Do, R.P.; Ponzi, D.; Stahlhut, R.W.; Drury, B.E.; Nagel, S.C.; Welshons, W.V.; Besch-Williford, C.L.; Palanza, P.; Parmigiani, S.; et al. Metabolic Disruption in Male Mice Due to Fetal Exposure to Low but Not High Doses of Bisphenol A (BPA): Evidence for Effects on Body Weight, Food Intake, Adipocytes, Leptin, Adiponectin, Insulin and Glucose Regulation. Reprod. Toxicol. 2013, 42, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Gaffen, S.L. IL-17 in Obesity and Adipogenesis. Cytokine Growth Factor Rev. 2010, 21, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Zhang, Q.; Ma, S.; Liu, S.; Chen, Z.; Mo, Z.; You, Z. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int. J. Mol. Sci. 2016, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Balasubramanian, K. Gestational Exposure to Di(2-Ethylhexyl) Phthalate (DEHP) Impairs Pancreatic β-Cell Function in F1 Rat Offspring. Toxicol. Lett. 2015, 232, 46–57. [Google Scholar] [CrossRef]
- Lin, J.-F.; Wu, S.; Huang, S.-S.; Lu, B.-Y.; Lin, S.-M.; Tsai, S.-K. Resveratrol Protects Left Ventricle by Increasing Adenylate Kinase and Isocitrate Dehydrogenase Activities in Rats with Myocardial Infarction. Chin. J. Physiol 2011, 54, 406–412. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Banerjee, K.K.; Vaidya, V.A.; Kolthur-Seetharam, U. Early Stress History Alters Serum Insulin-Like Growth Factor-1 and Impairs Muscle Mitochondrial Function in Adult Male Rats. J. Neuroendocr. 2016, 28. [Google Scholar] [CrossRef]
- Vargas, J.; Junco, M.; Gomez, C.; Lajud, N. Early Life Stress Increases Metabolic Risk, HPA Axis Reactivity, and Depressive-Like Behavior When Combined with Postweaning Social Isolation in Rats. PLoS ONE 2016, 11, e0162665. [Google Scholar] [CrossRef] [Green Version]
- Gehrand, A.L.; Hoeynck, B.; Jablonski, M.; Leonovicz, C.; Ye, R.; Scherer, P.E.; Raff, H. Sex Differences in Adult Rat Insulin and Glucose Responses to Arginine: Programming Effects of Neonatal Separation, Hypoxia, and Hypothermia. Physiol. Rep. 2016, 4, e12972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilchmann-Diounou, H.; Olier, M.; Lencina, C.; Riba, A.; Barretto, S.; Nankap, M.; Sommer, C.; Guillou, H.; Ellero-Simatos, S.; Guzylack-Piriou, L.; et al. Early Life Stress Induces Type 2 Diabetes-like Features in Ageing Mice. Brain Behav. Immun. 2019, 80, 452–463. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased Intestinal Permeability in Obese Mice: New Evidence in the Pathogenesis of Nonalcoholic Steatohepatitis. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, N.; Tang, L.; Jahangiri, A.; de Villiers, W.; Eckhardt, E. Elevated IgG Levels against Specific Bacterial Antigens in Obese Patients with Diabetes and in Mice with Diet-Induced Obesity and Glucose Intolerance. Metabolism 2012, 61, 1211–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The Cellular and Signaling Networks Linking the Immune System and Metabolism in Disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of High-Fat Diet on the Intestinal Microbiota and Small Intestinal Physiology before and after the Onset of Obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Treviño, L.S.; Wang, Q.; Walker, C.L. Phosphorylation of Epigenetic “Readers, Writers and Erasers”: Implications for Developmental Reprogramming and the Epigenetic Basis for Health and Disease. Prog. Biophys. Mol. Biol. 2015, 118, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [Green Version]
- Man, A.L.; Bertelli, E.; Rentini, S.; Regoli, M.; Briars, G.; Marini, M.; Watson, A.J.M.; Nicoletti, C. Age-Associated Modifications of Intestinal Permeability and Innate Immunity in Human Small Intestine. Clin. Sci. 2015, 129, 515–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.D.; Gonzaga, M.I.; Paiolo, J.C.; de Souza, C.A.; Stefanutto, N.A.; Omori, W.P.; Pinheiro, D.G.; Brisot-ti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella Copri and Bacteroides Vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 8, 1107. [Google Scholar] [CrossRef]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.H.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human Gut Microbes Impact Host Serum Metabolome and Insulin Sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Teixeira, T.F.S.; Souza, N.C.S.; Chiarello, P.G.; Franceschini, S.C.C.; Bressan, J.; Ferreira, C.L.L.F.; Peluzio, M. do C.G. Intestinal Permeability Parameters in Obese Patients Are Correlated with Metabolic Syndrome Risk Factors. Clin. Nutr. 2012, 31, 735–740. [Google Scholar] [CrossRef]
- Secondulfo, M.; de Magistris, L.; Sapone, A.; Di Monda, G.; Esposito, P.; Carratù, R. Intestinal Permeability and Diabetes Mellitus Type 2. Minerva Gastroenterol. Dietol. 1999, 45, 187–192. [Google Scholar] [PubMed]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; McDonnell, M.E.; Shin, H.; Rehman, Q.; Hasturk, H.; Apovian, C.M.; Nikolajczyk, B.S. Elevated Proinflammatory Cytokine Production by a Skewed T Cell Compartment Requires Monocytes and Promotes Inflammation in Type 2 Diabetes. J. Immunol. 2011, 186, 1162–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFuria, J.; Belkina, A.C.; Jagannathan-Bogdan, M.; Snyder-Cappione, J.; Carr, J.D.; Nersesova, Y.R.; Markham, D.; Strissel, K.J.; Watkins, A.A.; Zhu, M.; et al. B Cells Promote Inflammation in Obesity and Type 2 Diabetes through Regulation of T-Cell Function and an Inflammatory Cytokine Profile. Proc. Natl. Acad. Sci. USA 2013, 110, 5133–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmer, M.L.; Slack, E.; de Gottardi, A.; Lawson, M.A.E.; Hapfelmeier, S.; Miele, L.; Grieco, A.; Van Vlierberghe, H.; Fahrner, R.; Patuto, N.; et al. The Liver May Act as a Firewall Mediating Mutualism between the Host and Its Gut Commensal Microbiota. Sci. Transl. Med. 2014, 6, 237ra66. [Google Scholar] [CrossRef] [Green Version]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased Intestinal Permeability and Tight Junction Alterations in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Garidou, L.; Pomié, C.; Klopp, P.; Waget, A.; Charpentier, J.; Aloulou, M.; Giry, A.; Serino, M.; Stenman, L.; Lahtinen, S.; et al. The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORγt and Controls Metabolic Disease. Cell Metab. 2015, 22, 100–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, C.-P.; Park, A.; Yang, B.-G.; Yun, C.H.; Kwak, M.-J.; Lee, G.-W.; Kim, J.-H.; Jang, M.S.; Lee, E.-J.; Jeun, E.-J.; et al. Gut-Specific Delivery of T-Helper 17 Cells Reduces Obesity and Insulin Resistance in Mice. Gastroenterology 2017, 152, 1998–2010. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J.; et al. Interleukin-22 Alleviates Metabolic Disorders and Restores Mucosal Immunity in Diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation That Is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Kanno, E.; Maruyama, R. Blunted Autonomic Responses and Low-Grade Inflammation in Mongolian Adults Born at Low Birth Weight. Tohoku J. Exp. Med. 2016, 240, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Luo, S.; Li, Y.; Li, Y.; Zhu, Q.; Jiang, J.; Wu, C.; Shen, T. Gestational and Lactational Exposure to Low-Dose Bisphenol A Increases Th17 Cells in Mice Offspring. Environ. Toxicol. Pharm. 2016, 47, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Menard, S.; Guzylack-Piriou, L.; Leveque, M.; Braniste, V.; Lencina, C.; Naturel, M.; Moussa, L.; Sekkal, S.; Harkat, C.; Gaultier, E.; et al. Food Intolerance at Adulthood after Perinatal Exposure to the Endocrine Disruptor Bisphenol A. FASEB J. 2014, 28, 4893–4900. [Google Scholar] [CrossRef]
- Menard, S.; Guzylack-Piriou, L.; Lencina, C.; Leveque, M.; Naturel, M.; Sekkal, S.; Harkat, C.; Gaultier, E.; Olier, M.; Garcia-Villar, R.; et al. Perinatal Exposure to a Low Dose of Bisphenol A Impaired Systemic Cellular Immune Response and Predisposes Young Rats to Intestinal Parasitic Infection. PLoS ONE 2014, 9, e112752. [Google Scholar] [CrossRef]
- Khokhlova, E.V.; Smeianov, V.V.; Efimov, B.A.; Kafarskaia, L.I.; Pavlova, S.I.; Shkoporov, A.N. Anti-Inflammatory Properties of Intestinal Bifidobacterium Strains Isolated from Healthy Infants. Microbiol. Immunol. 2012, 56, 27–39. [Google Scholar] [CrossRef]
- Malaisé, Y.; Ménard, S.; Cartier, C.; Lencina, C.; Sommer, C.; Gaultier, E.; Houdeau, E.; Guzylack-Piriou, L. Consequences of Bisphenol a Perinatal Exposure on Immune Responses and Gut Barrier Function in Mice. Arch. Toxicol. 2018, 92, 347–358. [Google Scholar] [CrossRef]
- van Esterik, J.C.J.; Dollé, M.E.T.; Lamoree, M.H.; van Leeuwen, S.P.J.; Hamers, T.; Legler, J.; van der Ven, L.T.M. Programming of Metabolic Effects in C57BL/6JxFVB Mice by Exposure to Bisphenol A during Gestation and Lactation. Toxicology 2014, 321, 40–52. [Google Scholar] [CrossRef]
- Dallman, M.F. Stress-Induced Obesity and the Emotional Nervous System. Trends Endocrinol. Metab. 2010, 21, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Björntorp, P. Do Stress Reactions Cause Abdominal Obesity and Comorbidities? Obes. Rev. 2001, 2, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Epel, E.S. Stress, Eating and the Reward System. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The Perfect Storm: Obesity, Adipocyte Dysfunction, and Metabolic Consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal Separation as a Model of Brain-Gut Axis Dysfunction. Psychopharmacology 2011, 214, 71–88. [Google Scholar] [CrossRef]
- Mayer, E.A.; Naliboff, B.D.; Chang, L.; Coutinho, S.V.V. Stress and Irritable Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G519–G524. [Google Scholar] [CrossRef]
- Wood, J.D. Visceral Pain: Spinal Afferents, Enteric Mast Cells, Enteric Nervous System and Stress. Curr. Pharm. Des. 2011, 17, 1573–1575. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability--a New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.M. A Role for the Gut Microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The Immune System in Irritable Bowel Syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Hislop, I.G. Childhood Deprivation: An Antecedent of the Irritable Bowel Syndrome. Med. J. Aust. 1979, 1, 372–374. [Google Scholar] [CrossRef]
- Lowman, B.C.; Drossman, D.A.; Cramer, E.M.; McKee, D.C. Recollection of Childhood Events in Adults with Irritable Bowel Syndrome. J. Clin. Gastroenterol. 1987, 9, 324–330. [Google Scholar] [CrossRef]
- Videlock, E.J.; Adeyemo, M.; Licudine, A.; Hirano, M.; Ohning, G.; Mayer, M.; Mayer, E.A.; Chang, L. Childhood Trauma Is Associated with Hypothalamic-Pituitary-Adrenal Axis Responsiveness in Irritable Bowel Syndrome. Gastroenterology 2009, 137, 1954–1962. [Google Scholar] [CrossRef] [Green Version]
- Badedi, M.; Solan, Y.; Darraj, H.; Sabai, A.; Mahfouz, M.; Alamodi, S.; Alsabaani, A. Factors Associated with Long-Term Control of Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2109542. [Google Scholar] [CrossRef]
- Gulcan, E.; Taser, F.; Toker, A.; Korkmaz, U.; Alcelik, A. Increased Frequency of Prediabetes in Patients with Irritable Bowel Syndrome. Am. J. Med. Sci. 2009, 338, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, T.; Uemura, Y.; Jiao, S.; Wang, D.; Lin, Z.; Narita, Y.; Suzuki, M.; Hirosawa, N.; Ichihara, Y.; et al. Bisphenol A in Combination with TNF-Alpha Selectively Induces Th2 Cell-Promoting Dendritic Cells in Vitro with an Estrogen-like Activity. Cell Mol. Immunol. 2010, 7, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.R.; Arseneault, L.; Caspi, A.; Fisher, H.L.; Moffitt, T.E.; Odgers, C.L.; Pariante, C.; Ambler, A.; Dove, R.; Kepa, A.; et al. Childhood Victimization and Inflammation in Young Adulthood: A Genetically Sensitive Cohort Study. Brain Behav. Immun. 2018, 67, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Castagné, R.; Delpierre, C.; Kelly-Irving, M.; Campanella, G.; Guida, F.; Krogh, V.; Palli, D.; Panico, S.; Sacerdote, C.; Tumino, R.; et al. A Life Course Approach to Explore the Biological Embedding of Socioeconomic Position and Social Mobility through Circulating Inflammatory Markers. Sci. Rep. 2016, 6, 25170. [Google Scholar] [CrossRef] [Green Version]
- Castagné, R.; Kelly-Irving, M.; Campanella, G.; Guida, F.; Krogh, V.; Palli, D.; Panico, S.; Sacerdote, C.; Tumino, R.; Kleinjans, J.; et al. Biological Marks of Early-Life Socioeconomic Experience Is Detected in the Adult Inflammatory Transcriptome. Sci. Rep. 2016, 6, 38705. [Google Scholar] [CrossRef] [Green Version]
- Danese, A.; Caspi, A.; Williams, B.; Ambler, A.; Sugden, K.; Mika, J.; Werts, H.; Freeman, J.; Pariante, C.M.; Moffitt, T.E.; et al. Biological Embedding of Stress through Inflammation Processes in Childhood. Mol. Psychiatry 2011, 16, 244–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood Maltreatment Predicts Adult Inflammation in a Life-Course Study. Proc. Natl. Acad. Sci. USA 2007, 104, 1319–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danese, A.; Moffitt, T.E.; Harrington, H.; Milne, B.J.; Polanczyk, G.; Pariante, C.M.; Poulton, R.; Caspi, A. Adverse Childhood Experiences and Adult Risk Factors for Age-Related Disease: Depression, Inflammation, and Clustering of Metabolic Risk Markers. Arch. Pediatr. Adolesc. Med. 2009, 163, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Fraga, S.; Severo, M.; Ramos, E.; Kelly-Irving, M.; Silva, S.; Ribeiro, A.I.; Petrovic, D.; Barros, H.; Stringhini, S. Childhood Socioeconomic Conditions Are Associated with Increased Chronic Low-Grade Inflammation over Adolescence: Findings from the EPITeen Cohort Study. Arch. Dis. Child. 2020, 105, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Maurel, M.; Castagné, R.; Berger, E.; Bochud, M.; Chadeau-Hyam, M.; Fraga, S.; Gandini, M.; Hutri-Kähönen, N.; Jalkanen, S.; Kivimäki, M.; et al. Patterning of Educational Attainment across Inflammatory Markers: Findings from a Multi-Cohort Study. Brain Behav. Immun. 2020, 90, 303–310. [Google Scholar] [CrossRef]
- Berger, E.; Castagné, R.; Chadeau-Hyam, M.; Bochud, M.; d’Errico, A.; Gandini, M.; Karimi, M.; Kivimäki, M.; Krogh, V.; Marmot, M.; et al. Multi-Cohort Study Identifies Social Determinants of Systemic Inflammation over the Life Course. Nat. Commun. 2019, 10, 773. [Google Scholar] [CrossRef] [Green Version]
- Lacey, R.E.; Bartley, M.; Kelly-Irving, M.; Bevilacqua, L.; Iob, E.; Kelly, Y.; Howe, L.D. Adverse Childhood Experiences and Early Life Inflammation in the Avon Longitudinal Study of Parents and Children. Psychoneuroendocrinology 2020, 122, 104914. [Google Scholar] [CrossRef]
- Karimi, M.; Castagné, R.; Delpierre, C.; Albertus, G.; Berger, E.; Vineis, P.; Kumari, M.; Kelly-Irving, M.; Chadeau-Hyam, M. Early-Life Inequalities and Biological Ageing: A Multisystem Biological Health Score Approach in UnderstandingSociety. J. Epidemiol. Community Health 2019, 73, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Keane, J.M.; Khashan, A.S.; McCarthy, F.P.; Kenny, L.C.; Collins, J.M.; O’Donovan, S.M.; Brown, J.R.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; et al. Identifying a Biological Signature of Prenatal Maternal Stress. JCI Insight 2020. [Google Scholar] [CrossRef]
- Roque, A.; Ochoa-Zarzosa, A.; Torner, L. Maternal Separation Activates Microglial Cells and Induces an Inflammatory Response in the Hippocampus of Male Rat Pups, Independently of Hypothalamic and Peripheral Cytokine Levels. Brain Behav. Immun. 2016, 55, 39–48. [Google Scholar] [CrossRef]
- Roque, S.; Mesquita, A.R.; Palha, J.A.; Sousa, N.; Correia-Neves, M. The Behavioral and Immunological Impact of Maternal Separation: A Matter of Timing. Front. Behav. Neurosci. 2014, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Barreau, F.; Ferrier, L.; Fioramonti, J.; Bueno, L. Neonatal Maternal Deprivation Triggers Long Term Alterations in Colonic Epithelial Barrier and Mucosal Immunity in Rats. Gut 2004, 53, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, A.; Olier, M.; Lacroix-Lamandé, S.; Lencina, C.; Bacquié, V.; Harkat, C.; Van Langendonck, N.; Gillet, M.; Cartier, C.; Baron, M.; et al. Early Life Stress in Mice Is a Suitable Model for Irritable Bowel Syndrome but Does Not Predispose to Colitis nor Increase Susceptibility to Enteric Infections. Brain Behav. Immun. 2018, 73, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gareau, M.G.; Jury, J.; Yang, P.C.; MacQueen, G.; Perdue, M.H. Neonatal Maternal Separation Causes Colonic Dysfunction in Rat Pups Including Impaired Host Resistance. Pediatr. Res. 2006, 59, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riba, A.; Olier, M.; Lacroix-Lamandé, S.; Lencina, C.; Bacquié, V.; Harkat, C.; Gillet, M.; Baron, M.; Sommer, C.; Mallet, V.; et al. Paneth Cell Defects Induce Microbiota Dysbiosis in Mice and Promote Visceral Hypersensitivity. Gastroenterology 2017, 153, 1594–1606.e2. [Google Scholar] [CrossRef] [Green Version]
- Belsky, D.W.; Caspi, A.; Houts, R.; Cohen, H.J.; Corcoran, D.L.; Danese, A.; Harrington, H.; Israel, S.; Levine, M.E.; Schaefer, J.D.; et al. Quantification of Biological Aging in Young Adults. Proc. Natl. Acad. Sci. USA 2015, 112, E4104–E4110. [Google Scholar] [CrossRef] [Green Version]
- Vaiserman, A.; Lushchak, O. Developmental Origins of Type 2 Diabetes: Focus on Epigenetics. Ageing Res. Rev. 2019, 55, 100957. [Google Scholar] [CrossRef] [PubMed]
- Brulport, A.; Vaiman, D.; Chagnon, M.-C.; Le Corre, L. Obesogen Effect of Bisphenol S Alters MRNA Expression and DNA Methylation Profiling in Male Mouse Liver. Chemosphere 2020, 241, 125092. [Google Scholar] [CrossRef] [PubMed]
- Brehm, E.; Flaws, J.A. Transgenerational Effects of Endocrine-Disrupting Chemicals on Male and Female Reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Disease. Environ. Epigenet 2018, 4, dvy016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and Mechanisms of Epigenetic Inheritance in Animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Son, S.Y.; Lee, C.H. Perplexing Metabolomes in Fungal-Insect Trophic Interactions: A Terra Incognita of Mycobiocontrol Mechanisms. Front. Microbiol. 2016, 7, 1678. [Google Scholar] [CrossRef] [Green Version]
- Dolinoy, D.C.; Jirtle, R.L. Environmental Epigenomics in Human Health and Disease. Environ. Mol. Mutagen. 2008, 49, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, E.; Chiarelli, F. Endocrine-Disrupting Chemicals and Insulin Resistance in Children. Biomedicines 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Speidel, J.T.; Xu, M.; Abdel-Rahman, S.Z. Bisphenol A (BPA) and Bisphenol S (BPS) Alter the Promoter Activity of the ABCB1 Gene Encoding P-Glycoprotein in the Human Placenta in a Haplotype-Dependent Manner. Toxicol. Appl. Pharm. 2018, 359, 47–54. [Google Scholar] [CrossRef]
- Brulport, A.; Lencina, C.; Chagnon, M.-C.; Le Corre, L.; Guzylack-Piriou, L. Transgenerational Effects on Intestinal Inflammation Status in Mice Perinatally Exposed to Bisphenol S. Chemosphere 2021, 262, 128009. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Rashid, C.; Xin, F.; Li, C.; Polyak, E.; Duemler, A.; van der Meer, T.; Stefaniak, M.; Wajid, S.; Doliba, N.; et al. Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring. Environ. Health Perspect. 2017, 125, 097022. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Edwards, M.; Shetty, S.R.J.; Gatewood, J.D.; Taylor, J.A.; Rissman, E.F.; Connelly, J.J. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinology 2012, 153, 3828–3838. [Google Scholar] [CrossRef] [Green Version]
- Marjoram, L.; Alvers, A.; Deerhake, M.E.; Bagwell, J.; Mankiewicz, J.; Cocchiaro, J.L.; Beerman, R.W.; Willer, J.; Sumigray, K.D.; Katsanis, N.; et al. Epigenetic Control of Intestinal Barrier Function and Inflammation in Zebrafish. Proc. Natl. Acad. Sci. USA 2015, 112, 2770–2775. [Google Scholar] [CrossRef] [Green Version]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic Programming by Maternal Behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Kinnally, E.L.; Feinberg, C.; Kim, D.; Ferguson, K.; Leibel, R.; Coplan, J.D.; John Mann, J. DNA Methylation as a Risk Factor in the Effects of Early Life Stress. Brain Behav. Immun. 2011, 25, 1548–1553. [Google Scholar] [CrossRef] [Green Version]
- Provençal, N.; Suderman, M.J.; Guillemin, C.; Massart, R.; Ruggiero, A.; Wang, D.; Bennett, A.J.; Pierre, P.J.; Friedman, D.P.; Côté, S.M.; et al. The Signature of Maternal Rearing in the Methylome in Rhesus Macaque Prefrontal Cortex and T Cells. J. Neurosci. 2012, 32, 15626–15642. [Google Scholar] [CrossRef] [PubMed]
- Marioni, R.E.; Shah, S.; McRae, A.F.; Chen, B.H.; Colicino, E.; Harris, S.E.; Gibson, J.; Henders, A.K.; Redmond, P.; Cox, S.R.; et al. DNA Methylation Age of Blood Predicts All-Cause Mortality in Later Life. Genome Biol. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castagné, R.; Kelly-Irving, M.; Krogh, V.; Palli, D.; Panico, S.; Sacerdote, C.; Tumino, R.; Hebels, D.G.; Kleinjans, J.C.; de Kok, T.M.; et al. A Multi-Omics Approach to Investigate the Inflammatory Response to Life Course Socioeconomic Position. Epigenomics 2020, 12, 1287–1302. [Google Scholar] [CrossRef] [PubMed]
- van den Wijngaard, R.M.; Stanisor, O.I.; van Diest, S.A.; Welting, O.; Wouters, M.M.; Cailotto, C.; de Jonge, W.J.; Boeckxstaens, G.E. Susceptibility to Stress Induced Visceral Hypersensitivity in Maternally Separated Rats Is Transferred across Generations. Neurogastroenterol. Motil. 2013, 25, e780–e790. [Google Scholar] [CrossRef]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef]
- Mathis, D.; Shoelson, S.E. Immunometabolism: An Emerging Frontier. Nat. Rev. Immunol. 2011, 11, 81. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. IRS-1-Mediated Inhibition of Insulin Receptor Tyrosine Kinase Activity in TNF-Alpha- and Obesity-Induced Insulin Resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef]
- Shale, M.; Schiering, C.; Powrie, F. CD4(+) T-Cell Subsets in Intestinal Inflammation. Immunol. Rev. 2013, 252, 164–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollrath, J.; Powrie, F.M. Controlling the Frontier: Regulatory T-Cells and Intestinal Homeostasis. Semin. Immunol. 2013, 25, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Littman, D.R.; Rudensky, A.Y. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell 2010, 140, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Hebb, D.O. The American Revolution. Am. Psychol. 1960, 15, 735–745. [Google Scholar] [CrossRef]
- Bailoo, J.D.; Murphy, E.; Boada-Saña, M.; Varholick, J.A.; Hintze, S.; Baussière, C.; Hahn, K.C.; Göpfert, C.; Palme, R.; Voelkl, B.; et al. Effects of Cage Enrichment on Behavior, Welfare and Outcome Variability in Female Mice. Front. Behav. Neurosci. 2018, 12, 232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzylack-Piriou, L.; Ménard, S. Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?—An Overview. Biomolecules 2021, 11, 687. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11050687
Guzylack-Piriou L, Ménard S. Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?—An Overview. Biomolecules. 2021; 11(5):687. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11050687
Chicago/Turabian StyleGuzylack-Piriou, Laurence, and Sandrine Ménard. 2021. "Early Life Exposure to Food Contaminants and Social Stress as Risk Factor for Metabolic Disorders Occurrence?—An Overview" Biomolecules 11, no. 5: 687. https://0-doi-org.brum.beds.ac.uk/10.3390/biom11050687