Analysis of Differences in the Spatial Distribution among Terrestrial Mammals Using Geodetector—A Case Study of China
Abstract
:1. Introduction
2. Materials and Methods
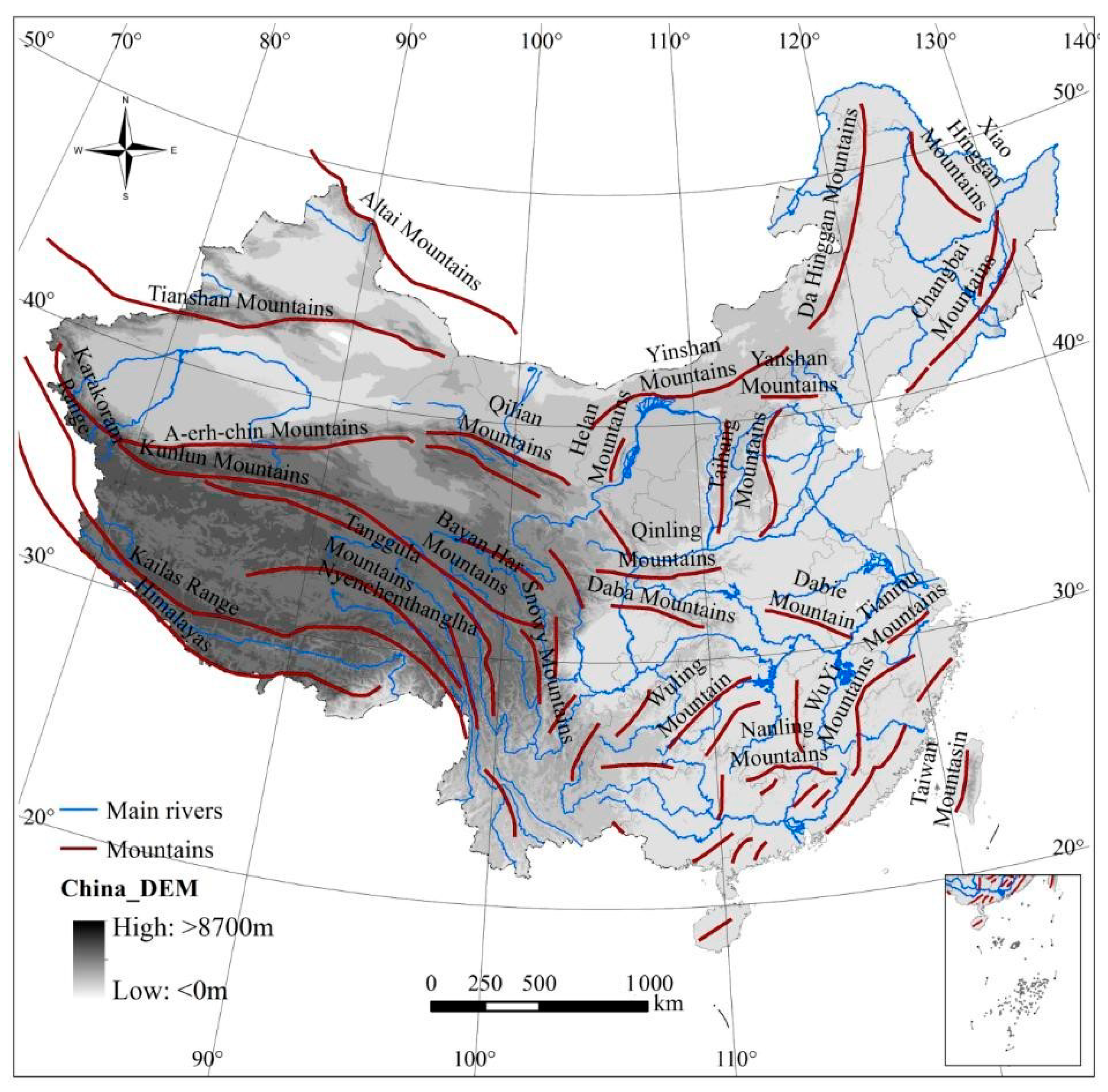
2.1. Study Area
2.2. Data Materials
2.2.1. Distribution Data of Terrestrial Mammals
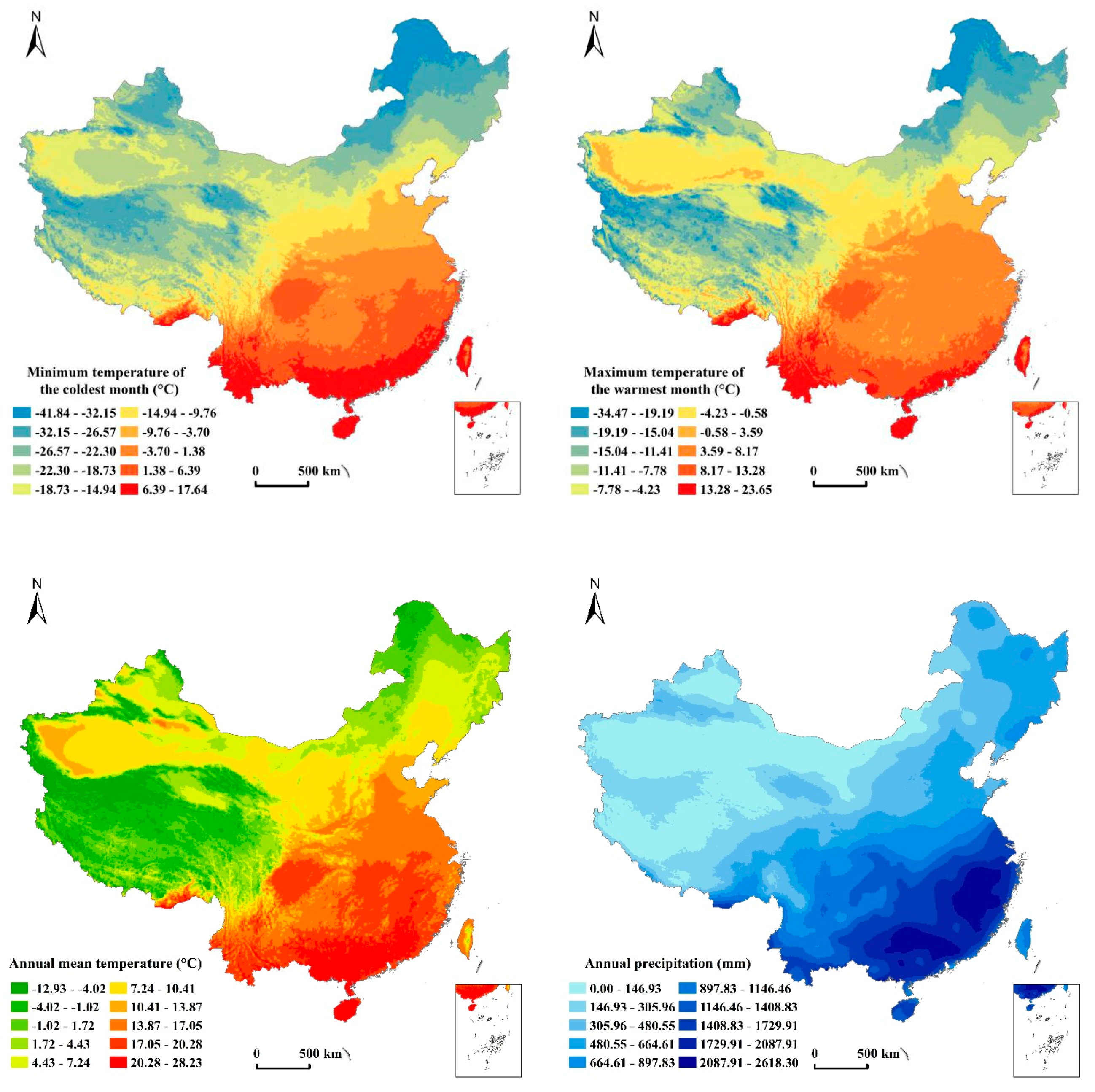
2.2.2. Data Resources of Environmental Factors
2.3. Methods
3. Results
3.1. Influencing Factors on the Spatial Distribution of Terrestrial Mammalian Richness
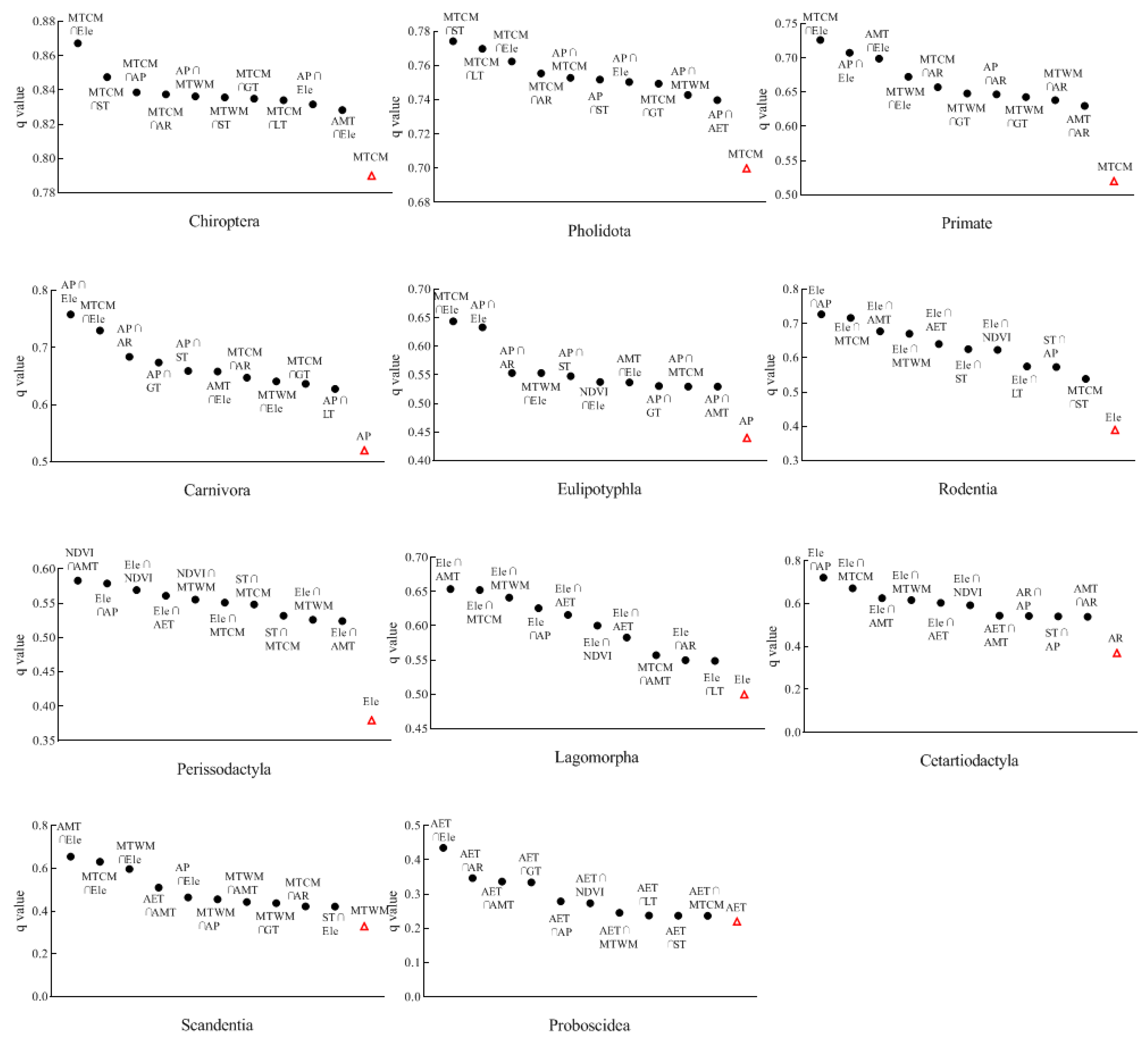
3.2. Influencing Factors on the Distribution of Mammalian Orders
3.3. Indication of Environment Factors on the Distribution of Mammal Richness
4. Discussion
5. Conclusions
- (1)
- The spatial pattern of terrestrial mammals in China showed a low east–west trend and distinct heterogeneity to the north and south. AP and MTCM were the dominant factors affecting the spatial differentiation of mammal richness in China.
- (2)
- The characteristics of the distribution of species richness across taxonomic groups were influenced by different environmental factors. Many mammalian orders were affected by regional freezing tolerance and productivity levels (mainly MTCM and AP). Perissodactyla was mainly influenced by habitat heterogeneity, while regional productivity levels had less impact on Lagomorpha.
- (3)
- Extremely low ambient temperatures had negative impacts on the distribution of animals, with too little precipitation not being conducive to the aggregation of many species. At a certain altitude, mammalian taxonomic richness decreased with increasing altitude. Fewer mammals were present in regions where the altitude was too flat, with most mammals occurring in forest land.
- (4)
- The interactions of any two environmental factors had remarkable bivariate enhancement or nonlinear enhancement effects on the spatial distribution of species richness with respect to individual variables. The synergies of elevation with the minimum temperature of the coldest month and annual precipitation can best explain the regional distribution differences in mammal richness in China.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scanes, C.G. Chapter 19—Human Activity and Habitat Loss: Destruction, Fragmentation, and Degradation. In Animals and Human Society; Scanes, C.G., Toukhsati, S.R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–482. [Google Scholar] [CrossRef]
- Danell, K.; Lundberg, P.; Niemela, P. Species richness in mammalian herbivores: Patterns in the boreal zone. Ecography 1996, 19, 404–409. [Google Scholar] [CrossRef]
- Medellín, R.A. Mammal diversity and conservation in the selva-lacandona, Chiapas, Mexico. Conserv. Biol. 1994, 8, 780–799. [Google Scholar] [CrossRef]
- Andrews, P.; O’Brien, E.M. Climate, vegetation, and predictable gradients in mammal species richness in southern Africa. J. Zool. 2000, 251, 205–231. [Google Scholar] [CrossRef]
- Badgley, C.; Fox, D.L. Ecological biogeography of North American mammals: Species density and ecological structure in relation to environmental gradients. J. Biogeogr. 2000, 27, 1437–1467. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.H. Two decades of homage to santa-rosalia—Toward a general-theory of diversity. Am. Zool. 1981, 21, 877–888. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.H. Species-energy theory—An extension of species-area theory. Oikos 1983, 41, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.R.G.; Lennon, J.J.; Lawrenson, J.A. British bird species distributions and the energy theory. Nature 1988, 335, 539–541. [Google Scholar] [CrossRef]
- Currie, D.J. Energy and large-scale patterns of animal-species and plant-species richness. Am. Nat. 1991, 137, 27–49. [Google Scholar] [CrossRef]
- Connell, J.H.; Orias, E. The ecological regulation of species diversity. Am. Nat. 1964, 98, 399–414. [Google Scholar] [CrossRef]
- Stevens, G.C. The latitudinal gradient in geographical range—How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- O’Brien, E.M. Climatic gradients in woody plant-species richness—Towards an explanation-based on an analysis of Southern Africa woody flora. J. Biogeogr. 1993, 20, 181–198. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Porter, E.E.; Diniz, J.A.F. Productivity and history as predictors of the latitudinal diversity gradient of terrestrial birds. Ecology 2003, 84, 1608–1623. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, B.A. Ecology’s oldest pattern? Trends Ecol. Evol. 2001, 16, 470. [Google Scholar] [CrossRef]
- Kerr, J.T.; Packer, L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 1997, 385, 252–254. [Google Scholar] [CrossRef]
- Ricklefs, R.E. Community diversity—Relative roles of local and regional processes. Science 1987, 235, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.D.; Ropero, R.F.; Aguilera, P.A.; Rumi, R.; Salmeron, A. Continuous Bayesian networks for the estimation of species richness. Prog. Artif. Intell. 2015, 4, 49–57. [Google Scholar] [CrossRef]
- Grenyer, R.; Orme, C.D.L.; Jackson, S.F.; Thomas, G.H.; Davies, R.G.; Davies, T.J.; Jones, K.E.; Olson, V.A.; Ridgely, R.S.; Rasmussen, P.C.; et al. Global distribution and conservation of rare and threatened vertebrates. Nature 2006, 444, 93–96. [Google Scholar] [CrossRef]
- Huntley, B.; Berry, P.M.; Cramer, W.; McDonald, A.P. Modelling present and potential future ranges of some European higher plants using climate response surfaces. J. Biogeogr. 1995, 22, 967–1001. [Google Scholar] [CrossRef] [Green Version]
- Vayssières, M.P.; Plant, R.E.; Allen-Diaz, B.H. Classification trees: An alternative non-parametric approach for predicting species distributions. J. Veg. Sci. 2000, 11, 679–694. [Google Scholar] [CrossRef]
- De’ath, G. Multivariate regression trees: A new technique for modeling species—Environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar] [CrossRef]
- Ostmann, A.; Arbizu, P.M. Predictive models using randomForest regression for distribution patterns of meiofauna in Icelandic waters. Mar. Biodivers. 2018, 48, 719–735. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2020, 1–14. [Google Scholar] [CrossRef]
- Aguilar, M.; Lado, C. Ecological niche models reveal the importance of climate variability for the biogeography of protosteloid amoebae. ISME J. 2012, 6, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.F.; Xu, C.D. Geodetector: Principle and prospective. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, T.L.; Fu, B.J. A measure of spatial stratified heterogeneity. Ecol. Indic. 2016, 67, 250–256. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, X.H.; Christakos, G.; Liao, Y.-L.; Zhang, T.; Gu, X.; Zheng, X.-Y. Geographical Detectors-Based Health Risk Assessment and its Application in the Neural Tube Defects Study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Chen, C.L.; Zhang, Q.J.; Lv, X.; Huang, X.J. Analysis on spatial-temporal characteristics and driving mechanisms of cropland occupation and supplement in Jiangsu Province. Econ. Geogr. 2016, 36, 155–163. [Google Scholar]
- Zou, B.; Xu, S.; Zhang, J. Spatial Variation Analysis of Urban Air Pollution Using GIS: A Land Use Perspective. Geomat. Inf. Sci. Wuhan Univ. 2017, 42, 216–222. [Google Scholar]
- Shen, J.; Zhang, N.; He, B.; Liu, C.Y.; Li, Y.; Zhang, H.Y.; Chen, X.Y.; Lin, H. Construction of a GeogDetector-based model system to indicate the potential occurrence of grasshoppers in Inner Mongolia steppe habitats. Bull. Entomol. Res. 2015, 105, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.X.; Wu, E.Q.; Liu, J.; Qu, X.C.; Liu, C.; Ning, B.A.; Liu, Y. Distribution Characteristics of Spermophilus dauricus in Manchuria City in China in 2015 through ‘3S’ Technology. Biomed. Environ. Sci. 2016, 29, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, W.; Liu, Y.; Zhang, Y.; Ma, W.; Xin, K.; Wang, M. Uncovering the relative influences of space and environment in shaping the biogeographic patterns of mangrove mollusk diversity. ICES J. Mar. Sci. 2019, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Xu, C.; Ma, J.; Zhang, H.; Xu, C. Sandwich mapping of rodent density in Jilin Province, China. J. Geogr. Sci. 2018, 28, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Jiao, Z.; Ma, J.; Gao, X.; Xiao, J.; Muhammad Abid Hayat, W.H. Modelling the potential distribution of arbovirus vector Aedes aegypti under current and future climate scenarios in Taiwan, China. Pest. Manag. Sci. 2019, 75, 3076–3083. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Gao, X.; Zheng, K.; Ma, J.; Jiao, Z.; Xiao, J.; Wang, H. The potential distribution and dynamics of important vectors Culex pipiens pallens and Culex pipiens quinquefasciatus in China under climate change scenarios: An ecological niche modelling approach. Pest. Manag. Sci. 2020, 76, 3096–3107. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Rao, M.; Kang, A.L.; Xie, Y. Climate change adaptation planning for biodiversity conservation: A review. Adv. Clim. Chang. Res. 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Zhang, R. Geological events and mammalian distribution in China. Acta Zool. Sin. 2002, 48, 141–153. [Google Scholar]
- Jiang, Z.; Ma, Y.; Wu, Y.; Wang, Y.; Zhou, K.; Liu, S.; Feng, Z. China’s Mammal Diversity and Geographical Distribution; China Science Publishing: Beijing, China, 2015. [Google Scholar]
- Turner, J.R.G.; Gatehouse, C.M.; Corey, C.A. Does solar-energy control organic diversity—Butterflies, moths and the British climate. Oikos 1987, 48, 195–205. [Google Scholar] [CrossRef]
- Abramsky, Z.; Rosenzweig, M.L. Tilman predicted productivity diversity relationship shown by desert rodents. Nature 1984, 309, 150–151. [Google Scholar] [CrossRef]
- Currie, D.J.; Paquin, V. Large-scale biogeographical patterns of species richness of trees. Nature 1987, 329, 326–327. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielborger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Jenks, G.F. The data model concept in statistical mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- Ding, Y.T.; Zhang, M.; Qian, X.Y.; Li, C.R.; Chen, S.; Wang, W.W. Using the geographical detector technique to explore the impact of socioeconomic factors on PM2.5 concentrations in China. J. Clean. Prod. 2019, 211, 1480–1490. [Google Scholar] [CrossRef]
- Zhu, L.; Meng, J.; Zhu, L. Applying Geodetector to disentangle the contributions of natural and anthropogenic factors to NDVI variations in the middle reaches of the Heihe River Basin. Ecol. Indic. 2020, 117, 106545. [Google Scholar] [CrossRef]
- Eronen, J.T.; Puolamaki, K.; Liu, L.; Lintulaakso, K.; Damuth, J.; Janis, C.; Fortelius, M. Precipitation and large herbivorous mammals I: Estimates from present-day communities. Evol. Ecol. Res. 2010, 12, 217–233. [Google Scholar]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.H.; Langridge, J.L. Predicting savanna vegetation structure on the basis of plant available moisture (PAM) and plant available nutrients (PAN): A case study from Australia. J. Biogeogr. 1997, 24, 813–825. [Google Scholar] [CrossRef]
- Witecha, M.J. Effects of Fire and Precipitation on Small Mammal Populations and Communities; Texas A&M University: Kingsville, TX, USA, 2011. [Google Scholar]
- Lin, X.; Wang, Z.; Tang, Z.; Zhao, S.; Fang, J. Geographic patterns and environmental correlates of terrestrial mammal species richness in China. Biodivers. Sci. 2009, 17, 652–663. [Google Scholar]
- Wang, Z.; Tang, Z.; Fan, J. The species-energy hypothesis as a mechanism for species richness patterns. Biodivers. Sci. 2009, 17, 613–624. [Google Scholar]
- Schap, J.A.; Samuels, J.X.; Joyner, T.A. Ecometric estimation of present and past climate of North America using crown heights of rodents and lagomorphs. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020. [Google Scholar] [CrossRef]
- Torres-Romero, E.J.; Olalla-Tarraga, M.A. Untangling human and environmental effects on geographical gradients of mammal species richness: A global and regional evaluation. J. Anim. Ecol. 2015, 84, 851–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulzer, E. Temperaturregulation bei Fledermäusen (Chiroptera) aus verschiedenen Klimazonen. Z. Vgl. Physiol. 1965, 50, 1–34. [Google Scholar] [CrossRef]
- Reeder, W.G.; Cowles, R.B. Aspects of Thermoregulation in Bats. J. Mammal. 1951, 32, 389–403. [Google Scholar] [CrossRef]
- Martin, R.D. Primates, Evolution of. In International Encyclopedia of the Social & Behavioral Sciences; Smelser, N.J., Baltes, P.B., Eds.; Pergamon: Oxford, UK, 2001; pp. 12032–12038. [Google Scholar] [CrossRef]
- Berkovitz, B.; Shellis, P. Chapter 12—Perissodactyla. In The Teeth of Mammalian Vertebrates; Berkovitz, B., Shellis, P., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 213–221. [Google Scholar] [CrossRef]
- He, J.; Pan, Z.; Liu, D.; Guo, X. Exploring the regional differences of ecosystem health and its driving factors in China. Sci. Total Environ. 2019, 673, 553–564. [Google Scholar] [CrossRef] [PubMed]





| AP | MTCM | MTWM | AET | NDVI | ST | LT | AMT | Ele | GT | AR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| q-statistic | 0.57 | 0.53 | 0.47 | 0.44 | 0.42 | 0.40 | 0.37 | 0.37 | 0.19 | 0.16 | 0.15 |
| p-value | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Effect direction | + | + | + | + | + | + | − | + | − | + | + |
| q(X2) | 0.57 | 0.53 | 0.47 | 0.44 | 0.42 | 0.40 | 0.37 | 0.37 | 0.19 | 0.16 | 0.15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| q(X1) | q(X1 ∩ X2) | AP | MTCM | MTWM | AET | NDVI | ST | LT | AMT | Ele | GT | AR |
| 0.57 | AP | bi-E | bi-E | bi-E | bi-E | bi-E | bi-E | bi-E | non-E | bi-E | bi-E | |
| 0.53 | MTCM | 0.66 | bi-E | bi-E | bi-E | bi-E | bi-E | bi-E | non-E | bi-E | non-E | |
| 0.47 | MTWM | 0.66 | 0.57 | bi-E | bi-E | bi-E | bi-E | bi-E | non-E | non-E | non-E | |
| 0.44 | AET | 0.62 | 0.58 | 0.57 | bi-E | bi-E | bi-E | bi-E | non-E | bi-E | bi-E | |
| 0.42 | NDVI | 0.61 | 0.63 | 0.63 | 0.56 | bi-E | bi-E | bi-E | bi-E | bi-E | non-E | |
| 0.40 | ST | 0.69 | 0.66 | 0.64 | 0.60 | 0.60 | bi-E | bi-E | non-E | bi-E | non-E | |
| 0.37 | LT | 0.66 | 0.65 | 0.63 | 0.56 | 0.51 | 0.55 | bi-E | bi-E | bi-E | bi-E | |
| 0.37 | AMT | 0.63 | 0.64 | 0.57 | 0.54 | 0.56 | 0.59 | 0.57 | non-E | non-E | non-E | |
| 0.19 | Ele | 0.80 | 0.80 | 0.73 | 0.67 | 0.59 | 0.61 | 0.54 | 0.74 | non-E | non-E | |
| 0.16 | GT | 0.69 | 0.68 | 0.66 | 0.55 | 0.56 | 0.55 | 0.45 | 0.63 | 0.47 | bi-E | |
| 0.15 | AR | 0.70 | 0.69 | 0.67 | 0.56 | 0.59 | 0.58 | 0.48 | 0.64 | 0.48 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.; Qian, T.; Sheng, C.; Xi, C.; Wang, J. Analysis of Differences in the Spatial Distribution among Terrestrial Mammals Using Geodetector—A Case Study of China. ISPRS Int. J. Geo-Inf. 2021, 10, 21. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi10010021
Chi Y, Qian T, Sheng C, Xi C, Wang J. Analysis of Differences in the Spatial Distribution among Terrestrial Mammals Using Geodetector—A Case Study of China. ISPRS International Journal of Geo-Information. 2021; 10(1):21. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi10010021
Chicago/Turabian StyleChi, Yao, Tianlu Qian, Caiying Sheng, Changbai Xi, and Jiechen Wang. 2021. "Analysis of Differences in the Spatial Distribution among Terrestrial Mammals Using Geodetector—A Case Study of China" ISPRS International Journal of Geo-Information 10, no. 1: 21. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi10010021






