Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.)
Abstract
:1. Introduction
- (i)
- Compared the performance of SRS-EFAs as predictors in SDMs, separately and combined with climate data, to determine the current potential distribution of the thirteen target species;
- (ii)
- Identified the most suitable potential areas for the conservation of vulnerable NDE oak species by comparing the spatial projections of habitat suitability derived from SDMs based on different sets of predictors;
- (iii)
- Depict the fittest biogeographic areas for oak conservation based on a species-richness map obtained through stacking individual SDM’s.
2. Materials and Methods
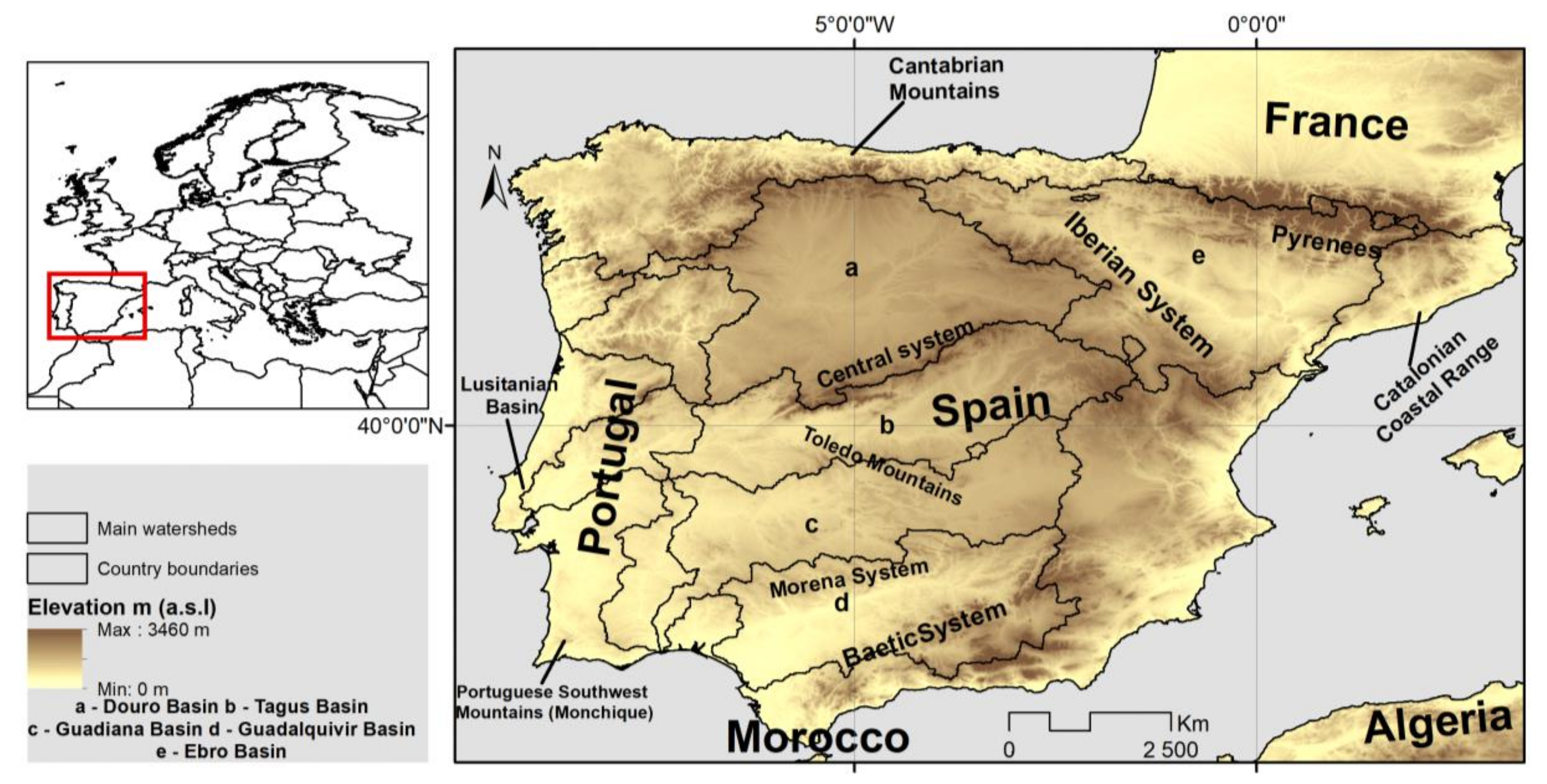
2.1. Study Area
2.2. Occurrence Data and Focal Taxa
2.3. Predictor Variables
2.3.1. Satellite-Based Ecosystem Functioning Attributes (EFAs)
2.3.2. Climatic Data
2.4. Model Development, Evaluation and Multi-Algorithm Ensembling
3. Results and Discussion
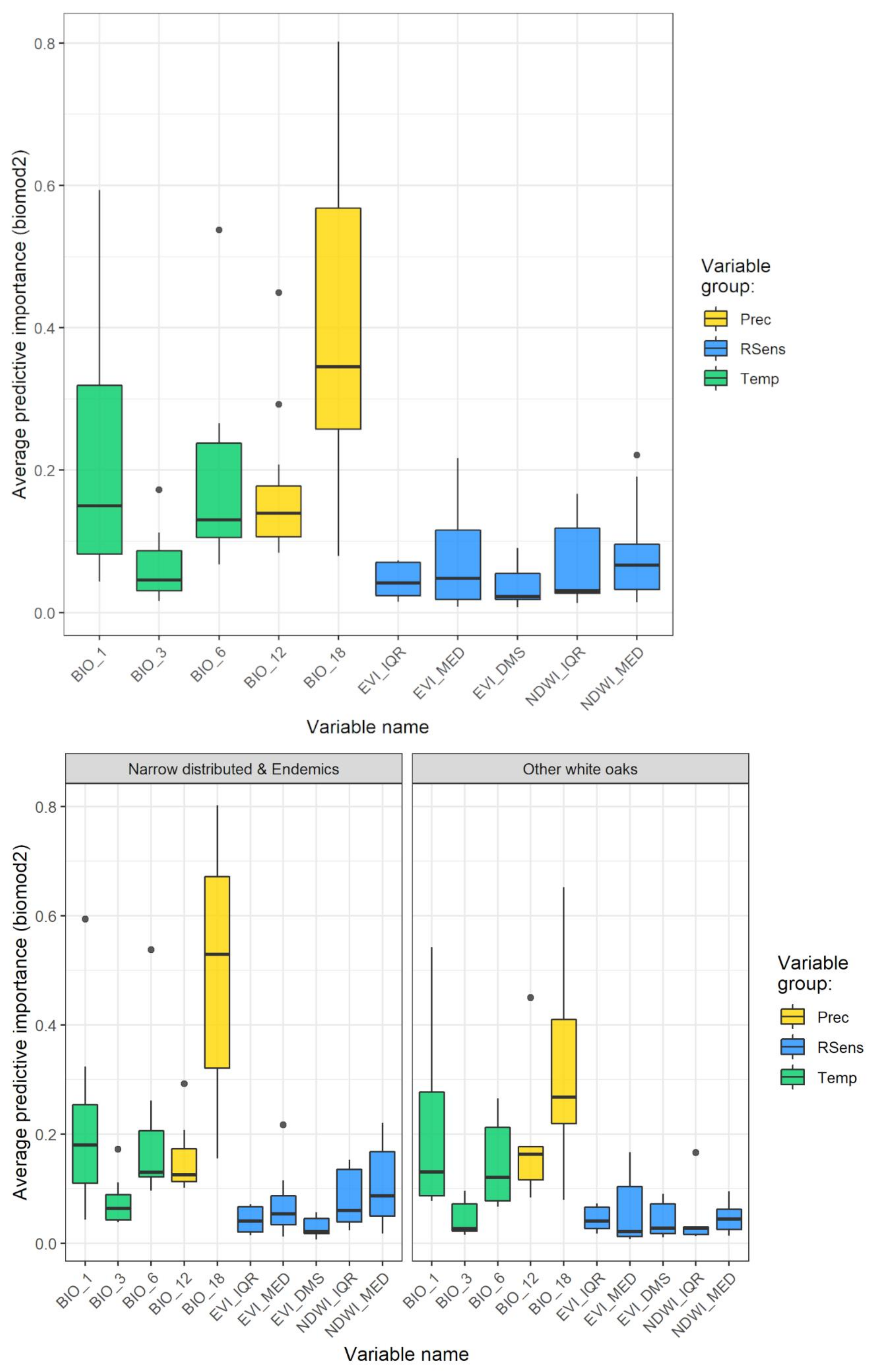
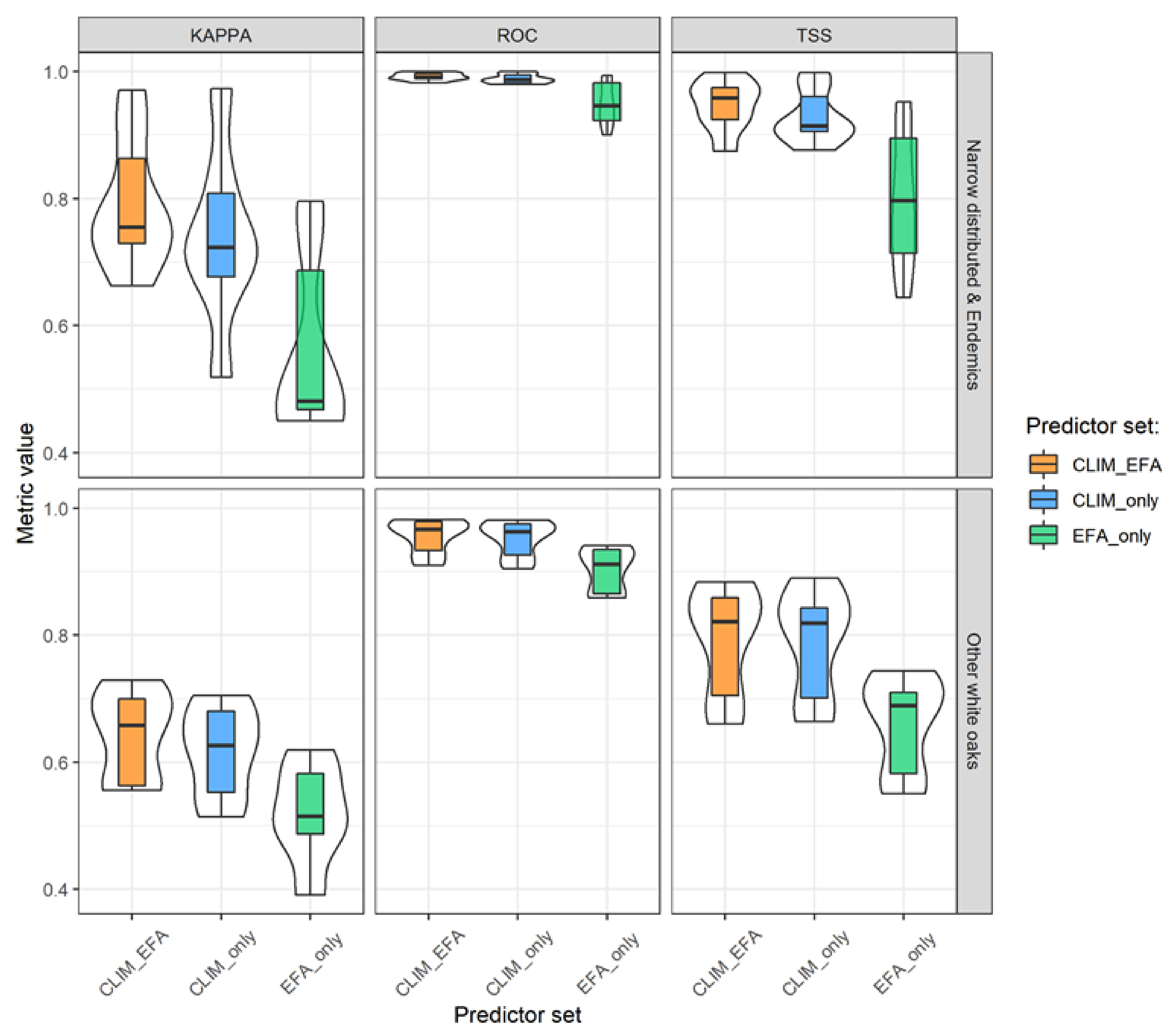
3.1. Model Performance and Added Value of EFAs as Predictors
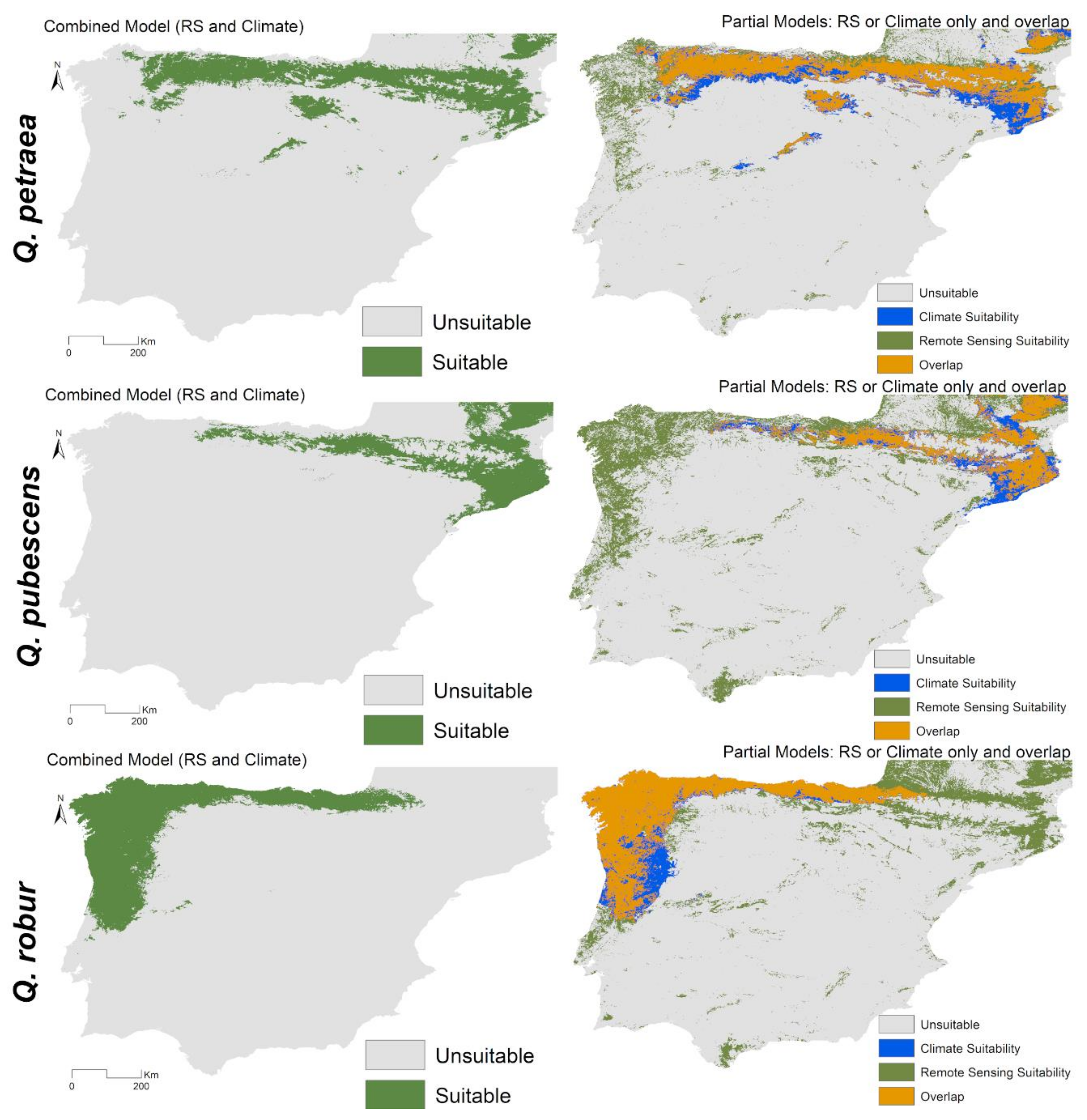
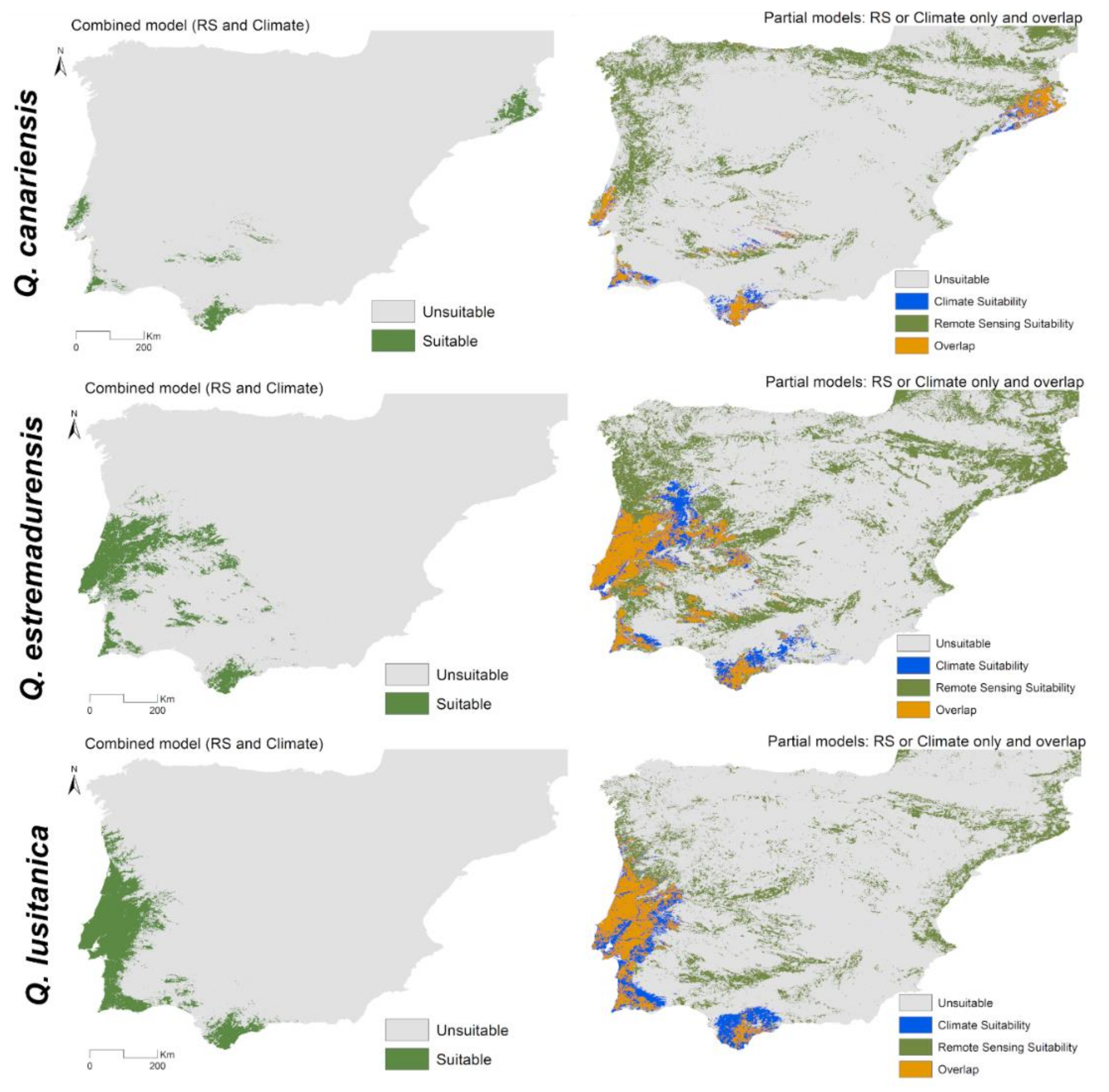
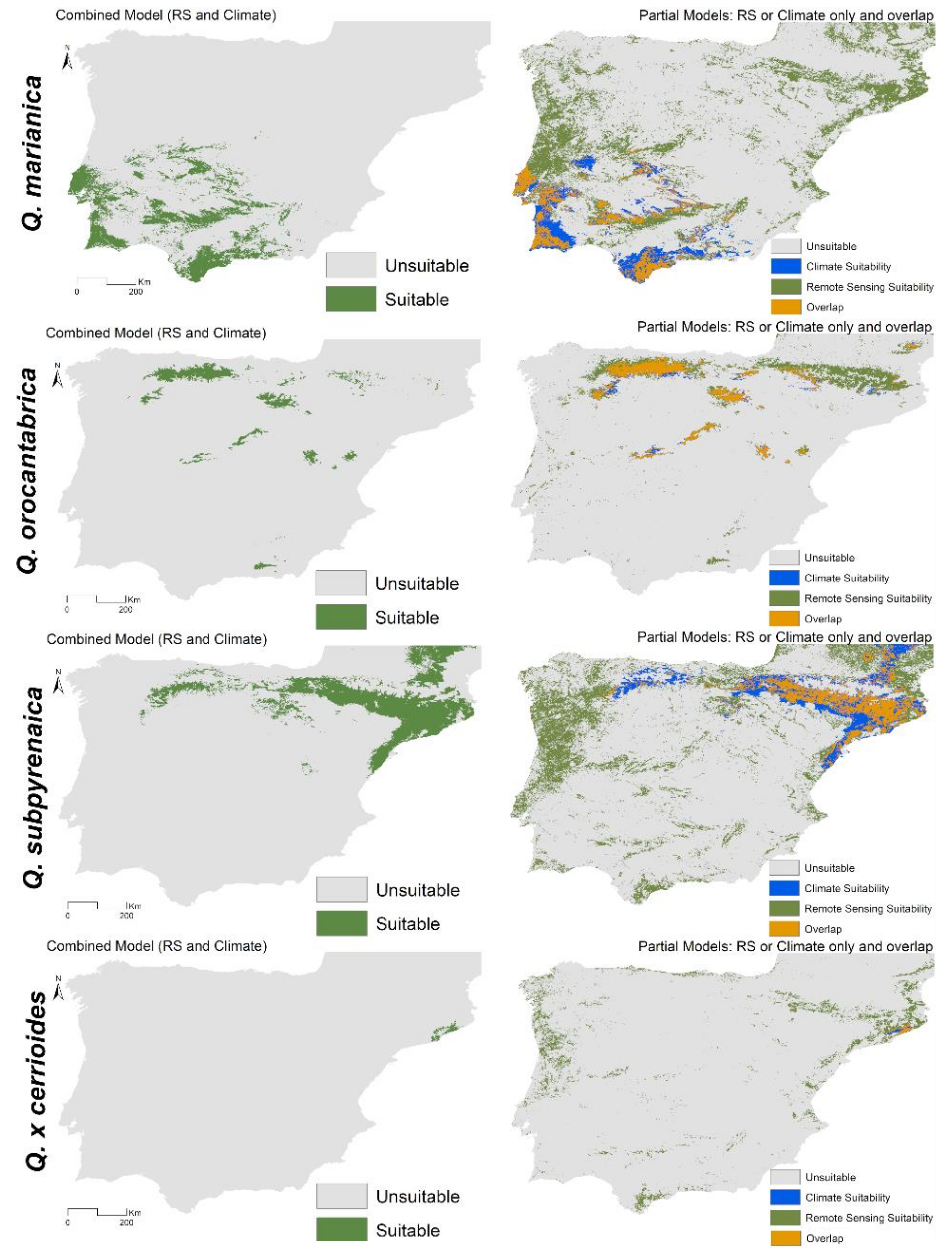
3.2. Combined Models for Individual Species
3.3. Spatial Projections and Patterns of Species Richness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Manos, P.S.; Doyle, J.J.; Nixon, K.C. Phylogeny, biogeography, and processes of molecular differentiation in Quercus subgenus Quercus (Fagaceae). Mol. Phylogenet. Evol. 1999, 12, 333–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatini, F.M.; Burrascano, S.; Keeton, W.S.; Levers, C.; Lindner, M.; Pötzschner, F.; Verkerk, P.J.; Bauhus, J.; Buchwald, E.; Chaskovsky, O. Where are Europe’s last primary forests? Divers. Distrib. 2018, 24, 1426–1439. [Google Scholar] [CrossRef] [Green Version]
- Anderegg, W.R.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J. Climate-driven risks to the climate mitigation potential of forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef] [PubMed]
- Neophytou, C.; Aravanopoulos, F.A.; Fink, S.; Dounavi, A. Detecting interspecific and geographic differentiation patterns in two interfertile oak species (Quercus petraea (Matt.) Liebl. and Q. robur L.) using small sets of microsatellite markers. For. Ecol. Manag. 2010, 259, 2026–2035. [Google Scholar] [CrossRef] [Green Version]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.-P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Balzan, M.V.; Hassoun, A.E.R.; Aroua, N.; Baldy, V.; Bou Dagher, M.; Branquinho, C.; Dutay, J.C.; El Bour, M.; Médail, F.; Mojtahid, M.; et al. Ecosystems; Union for the Mediterranean, Plan Bleu, UNEP/MAP: Marseille, France, 2020; p. 151. [Google Scholar]
- Franco, J.A. Quercus L. In Flora Iberica, (Plantanaceae-Plumbaginaceae [Partim]); Castroviejo, S., Ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 1990; Volume 2, pp. 15–36. [Google Scholar]
- Schwarz, O. Quercus L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1964; Volume 1, pp. 61–64. [Google Scholar]
- Rivas-Martínez, S.; Saénz, C. Enumeración de los Quercus de la Península Ibérica. Rivasgodaya 1991, 6, 101–110. [Google Scholar]
- Coutinho, A.X.P. Os Quercus de Portugal. Boletim Sociedade Broteriana 1888, 6, 47–116. [Google Scholar]
- Olalde, M.; Herrán, A.; Espinel, S.; Goicoechea, P.G. White oaks phylogeography in the Iberian Peninsula. For. Ecol. Manag. 2002, 156, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Denk, T.; Grimm, G.W.; Manos, P.S.; Deng, M.; Hipp, A.L. An updated infrageneric classification of the oaks: Review of previous taxonomic schemes and synthesis of evolutionary patterns. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–38. [Google Scholar]
- Rivas-Martínez, S.; Penas, A.; del Río, S.; González, T.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Utrecht, The Netherlands, 2017; pp. 29–80. [Google Scholar]
- De Dios, R.S.; Benito-Garzón, M.; Sainz-Ollero, H. Present and future extension of the Iberian submediterranean territories as determined from the distribution of marcescent oaks. Plant Ecol. 2009, 204, 189–205. [Google Scholar] [CrossRef]
- Del Río, S.; Penas, Á. Potential distribution of semi-deciduous forests in Castile and Leon (Spain) in relation to climatic variations. Plant Ecol. 2006, 185, 269–282. [Google Scholar]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Lomba, A.; Silva, R.; Vázquez, F.M.; García, C. Late Quaternary range shifts of marcescent oaks unveil the dynamics of a major biogeographic transition in southern Europe. Sci. Rep. 2020. Accepted. [Google Scholar]
- IPCC. Summary for Policymakers. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- García-Valdés, R.; Svenning, J.C.; Zavala, M.A.; Purves, D.W.; Araujo, M.B. Evaluating the combined effects of climate and land-use change on tree species distributions. J. Appl. Ecol. 2015, 52, 902–912. [Google Scholar] [CrossRef]
- Senf, C.; Seidl, R. Mapping the coupled human and natural disturbance regimes of Europe’s forests. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- McVay, J.D.; Hipp, A.L.; Manos, P.S. A genetic legacy of introgression confounds phylogeny and biogeography in oaks. Proc. R. Soc. B 2017, 284, 20170300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hipp, A.L. Should hybridization make us skeptical of the oak phylogeny. Int. Oak J. 2015, 26, 9–18. [Google Scholar]
- Vicioso, C. Revisión del Género Quercus en España; Tipografía Artítica: Madrid, Spain, 1950; p. 194. [Google Scholar]
- Vasconcellos, J.C.; Franco, J.A. Os Carvalhos de Portugal. Anais Instituto Superior Agronomia 1954, 21, 1–135. [Google Scholar]
- del Huget Villar, E. Estudios sobre los Quercus del Oeste mediterráneo. Anales Instituto Botanico A.J. Cavanilles 1958, 15, 3–114. [Google Scholar]
- Schwarz, O. Einige neue Eichen des Mediterrangebiets und Vorderasiens. Notizblatt Königl. botanischen Gartens Museums Berlin 1935, 114, 461–469. [Google Scholar] [CrossRef]
- Montserrat, P. Algunos aspectos de la diferenciación sistemática de los Quercus ibéricos. In Simposio de Biogeografía Ibérica; Instituto de Biologia Aplicada: Barcelona, Spain, 1957; Volume 26, pp. 61–75. [Google Scholar]
- Vila-Viçosa, C.; Vázquez, F.M.; Meireles, C.; Pinto-Gomes, C. Taxonomic peculiarities of marcescent oaks (Quercus, Fagaceae) in Southern Portugal. Lazaroa 2014, 35, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, O. Sobre los Quercus catalanes del subgénero Lepidobalanus Oerst. Cavanillesia 1936, 8, 65–100. [Google Scholar]
- Rivas-Martínez, S.; González-Días, T.E.; González, F.F.; Loidi, J.J.; Lousã, M.F.; Merino, Á.P. Vascular plant communities of Spain and Portugal: Addenda to the syntaxonomical checklist of 2001. Part II. Itinera Geobot. 2002, 15, 433–922. [Google Scholar]
- Rodríguez-Sánchez, F.; Hampe, A.; Jordano, P.; Arroyo, J. Past tree range dynamics in the Iberian Peninsula inferred through phylogeography and palaeodistribution modelling: A review. Rev. Palaeobot. Palynol. 2010, 162, 507–521. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R.; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Luque, S.; Pettorelli, N.; Vihervaara, P.; Wegmann, M. Improving biodiversity monitoring using satellite remote sensing to provide solutions towards the 2020 conservation targets. Methods Ecol. Evol. 2018, 9, 1784–1786. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz-Segura, D.; Cabello, J.; Paruelo, J.M.; Delibes, M. Use of descriptors of ecosystem functioning for monitoring a national park network: A remote sensing approach. Environ. Manag. 2009, 43, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, D.; Paruelo, J.; Cabello, J. Identification of current ecosystem functional types in the Iberian Peninsula. Glob. Ecol. Biogeogr. 2006, 15, 200–212. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Regos, A.; Gonçalves, J.F.; Alcaraz-Segura, D.; Honrado, J. Remotely sensed variables of ecosystem functioning support robust predictions of abundance patterns for rare species. Remote Sens. 2019, 11, 2086. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Castro, S.; Gonçalves, J.; Alves, P.; Alcaraz-Segura, D.; Honrado, J.P. Assessing the multi-scale predictive ability of ecosystem functional attributes for species distribution modelling. PLoS ONE 2018, 13, e0199292. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz-Segura, D.; Lomba, A.; Sousa-Silva, R.; Nieto-Lugilde, D.; Alves, P.; Georges, D.; Vicente, J.R.; Honrado, J.P. Potential of satellite-derived ecosystem functional attributes to anticipate species range shifts. Int. J. Appl. Earth Obs. Geoinf. 2017, 57, 86–92. [Google Scholar] [CrossRef]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.; Cardoso, A. Essential biodiversity variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.; Alves, P.; Pôças, I.; Marcos, B.; Sousa-Silva, R.; Lomba, Â.; Honrado, J.P. Exploring the spatiotemporal dynamics of habitat suitability to improve conservation management of a vulnerable plant species. Biodivers. Conserv. 2016, 25, 2867–2888. [Google Scholar] [CrossRef]
- Regos, A.; Gómez-Rodríguez, P.; Arenas-Castro, S.; Tapia, L.; Vidal, M.; Domínguez, J. Model-Assisted Bird Monitoring Based on Remotely Sensed Ecosystem Functioning and Atlas Data. Remote Sens. 2020, 12, 2549. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The effect of climate change on the richness distribution pattern of oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Lambraño, R.E.; de la Cruz, D.R.; Sánchez-Agudo, J.Á. Spatial oak decline models to inform conservation planning in the Central-Western Iberian Peninsula. For. Ecol. Manag. 2019, 441, 115–126. [Google Scholar] [CrossRef]
- Vessella, F.; López-Tirado, J.; Simeone, M.C.; Schirone, B.; Hidalgo, P.J. A tree species range in the face of climate change: Cork oak as a study case for the Mediterranean biome. Eur. J. For. Res. 2017, 136, 555–569. [Google Scholar] [CrossRef]
- Matías, L.; Abdelaziz, M.; Godoy, O.; Gómez-Aparicio, L. Disentangling the climatic and biotic factors driving changes in the dynamics of Quercus suber populations across the species latitudinal range. Divers. Distrib. 2019, 25, 524–535. [Google Scholar] [CrossRef] [Green Version]
- Noce, S.; Collalti, A.; Santini, M. Likelihood of changes in forest species suitability, distribution, and diversity under future climate: The case of Southern Europe. Ecol. Evol. 2017, 7, 9358–9375. [Google Scholar] [CrossRef]
- Ülker, E.D.; Tavşanoğlu, Ç.; Perktaş, U. Ecological niche modelling of pedunculate oak (Quercus robur) supports the ‘expansion–contraction’model of Pleistocene biogeography. Biol. J. Linn. Soc. 2017, 123, 338–347. [Google Scholar] [CrossRef]
- López-Tirado, J.; Hidalgo, P.J. Predicting suitability of forest dynamics to future climatic conditions: The likely dominance of Holm oak [Quercus ilex subsp. ballota (Desf.) Samp.] and Aleppo pine (Pinus halepensis Mill.). Ann. For. Sci. 2018, 75, 19. [Google Scholar] [CrossRef] [Green Version]
- Duque-Lazo, J.; Navarro-Cerrillo, R.; Ruíz-Gómez, F. Assessment of the future stability of cork oak (Quercus suber L.) afforestation under climate change scenarios in Southwest Spain. For. Ecol. Manag. 2018, 409, 444–456. [Google Scholar] [CrossRef]
- Ahrends, A.; Rahbek, C.; Bulling, M.T.; Burgess, N.D.; Platts, P.J.; Lovett, J.C.; Kindemba, V.W.; Owen, N.; Sallu, A.N.; Marshall, A.R. Conservation and the botanist effect. Biol. Conserv. 2011, 144, 131–140. [Google Scholar] [CrossRef]
- Coleman, C.O.; Radulovici, A.E. Challenges for the future of taxonomy: Talents, databases and knowledge growth. Megataxa 2020, 1, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Ely, C.V.; de Loreto Bordignon, S.A.; Trevisan, R.; Boldrini, I.I. Implications of poor taxonomy in conservation. J. Nat. Conserv. 2017, 36, 10–13. [Google Scholar]
- Loidi, J. Introduction to the Iberian Peninsula, General Features: Geography, Geology, Name, Brief History, Land Use and Conservation. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–27. [Google Scholar]
- Aedo, C.; Buira, A.; Medina, L.; Fernández-Albert, M. The Iberian Vascular Flora: Richness, Endemicity and Distribution Patterns. In The Vegetation of the Iberian Peninsula; Loidi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 101–130. [Google Scholar]
- Médail, F.; Baumel, A. Using phylogeography to define conservation priorities: The case of narrow endemic plants in the Mediterranean Basin hotspot. Biol. Conserv. 2018, 224, 258–266. [Google Scholar] [CrossRef]
- Endlicher, S. Generum Plantarum Supplementum Quartum; Fr. Beck: Vienna, Austria, 1847; p. 95. [Google Scholar] [CrossRef]
- Tschan, G.F.; Denk, T. Trichome types, foliar indumentum and epicuticular wax in the Mediterranean gall oaks, Quercus subsection Galliferae (Fagaceae): Implications for taxonomy, ecology and evolution. Bot. J. Linn. Soc. 2012, 169, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Gürke, M. Quercus. In Plantae Europaeae [Enumeratio Systematica et Synonymica Plantarum Phanerogamicarum in Europa Sponte Crescentium vel mere Inquilinarum]; Richter, K., Ed.; Engelmann: Leipzig, Germany, 1897; Volume 2, pp. 54–72. [Google Scholar]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Gonçalves, J.; Honrado, J.; Silva, R.; Vázquez, F.; Stephan, J.; García, C. Past, Present, and Future of Marcescent Mediterranean Forests. Biodivers. Inf. Sci. Stand. 2019, 3, e37195. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Marcos, B.; García, C.; Gonçalves, J. Biogeography from space—A new tool to disentangle Iberian Forests Ecology and Distribution. In Proceedings of the 1st Meeting of the Iberian Ecological Society & XIV AEET Meeting (SIBECOL 2019), Barcelona, Spain, 6 February 2019; p. 22. [Google Scholar]
- Team, R.C. R Version 3.5.3: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 1 May 2020).
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.; Anderson, R.; Dudık, M.; Ferrier, S. Novel methods improve prediction of species’ distribution models. Ecography 2006, 32, 66–77. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards Jr, T.C.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv Preprint 2014, arXiv:1406.5823. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Veloz, S.D.; Williams, J.W.; Blois, J.L.; He, F.; Otto-Bliesner, B.; Liu, Z. No-analog climates and shifting realized niches during the late quaternary: Implications for 21st-century predictions by species distribution models. Glob. Chang. Biol. 2012, 18, 1698–1713. [Google Scholar] [CrossRef]
- Vicente, J.R.; Gonçalves, J.; Honrado, J.P.; Randin, C.F.; Pottier, J.; Broennimann, O.; Lomba, A.; Guisan, A. A framework for assessing the scale of influence of environmental factors on ecological patterns. Ecol. Complex. 2014, 20, 151–156. [Google Scholar] [CrossRef]
- Civantos, E.; Monteiro, A.T.; Gonçalves, J.; Marcos, B.; Alves, P.; Honrado, J.P. Patterns of landscape seasonality influence passerine diversity: Implications for conservation management under global change. Ecol. Complex. 2018, 36, 117–125. [Google Scholar] [CrossRef]
- Honrado, J.P.; Pereira, H.M.; Guisan, A. Fostering integration between biodiversity monitoring and modelling. J. Appl. Ecol. 2016, 53, 1299–1304. [Google Scholar] [CrossRef]
- Cabello, J.; Fernández, N.; Alcaraz-Segura, D.; Oyonarte, C.; Pineiro, G.; Altesor, A.; Delibes, M.; Paruelo, J.M. The ecosystem functioning dimension in conservation: Insights from remote sensing. Biodivers. Conserv. 2012, 21, 3287–3305. [Google Scholar] [CrossRef] [Green Version]
- Bradley, B.A.; Olsson, A.D.; Wang, O.; Dickson, B.G.; Pelech, L.; Sesnie, S.E.; Zachmann, L.J. Species detection vs. habitat suitability: Are we biasing habitat suitability models with remotely sensed data? Ecol. Model. 2012, 244, 57–64. [Google Scholar] [CrossRef]
- McPherson, J.M.; Jetz, W.; Rogers, D.J. Using coarse-grained occurrence data to predict species distributions at finer spatial resolutions—Possibilities and limitations. Ecol. Model. 2006, 192, 499–522. [Google Scholar] [CrossRef]
- Fernandes, R.F.; Vicente, J.R.; Georges, D.; Alves, P.; Thuiller, W.; Honrado, J.P. A novel downscaling approach to predict plant invasions and improve local conservation actions. Biol. Invasions 2014, 16, 2577–2590. [Google Scholar] [CrossRef]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Lassueur, T.; Joost, S.; Randin, C.F. Very high resolution digital elevation models: Do they improve models of plant species distribution? Ecol. Model. 2006, 198, 139–153. [Google Scholar] [CrossRef]
- Pôças, I.; Gonçalves, J.; Marcos, B.; Alonso, J.; Castro, P.; Honrado, J.P. Evaluating the fitness for use of spatial data sets to promote quality in ecological assessment and monitoring. Int. J. Geogr. Inf. Sci. 2014, 28, 2356–2371. [Google Scholar] [CrossRef]
- Vila-Viçosa, C.; Vázquez, F.M.; Mendes, P.; Del Rio, S.; Musarella, C.; Cano-Ortiz, A.; Meireles, C. Syntaxonomic update on the relict groves of Mirbeck’s oak (Quercus canariensis Willd. and Q. marianica C. Vicioso) in southern Iberia. Plant Biosyst. 2015, 149, 512–526. [Google Scholar] [CrossRef]
- Ward, A. Fog at North Front, Gibraltar. Meteorol. Mag 1952, 81, 272–279. [Google Scholar]
- Wheeler, D. Factors governing sunshine in south-west Iberia: A review of western Europe’s sunniest region. Weather 2001, 56, 189–197. [Google Scholar] [CrossRef]
- Soriano, P.; Costa, M. The Coastal Levantine Area. In The Vegetation of the Iberian Peninsula; Springer: Berlin/Heidelberg, Germany, 2017; pp. 589–625. [Google Scholar]
- Anderson, E. Hybridization of the habitat. Evolution 1948, 2, 1–9. [Google Scholar] [CrossRef]
- Anderson, E.; Stebbins, G.L., Jr. Hybridization as an evolutionary stimulus. Evolution 1954, 8, 378–388. [Google Scholar] [CrossRef]
- Löf, M.; Castro, J.; Engman, M.; Leverkus, A.B.; Madsen, P.; Reque, J.A.; Villalobos, A.; Gardiner, E.S. Tamm Review: Direct seeding to restore oak (Quercus spp.) forests and woodlands. For. Ecol. Manag. 2019, 448, 474–489. [Google Scholar] [CrossRef]
- Safford, H.D.; Vallejo, V.R. Ecosystem management and ecological restoration in the Anthropocene: Integrating global change, soils, and disturbance in boreal and Mediterranean forests. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 36, pp. 259–308. [Google Scholar]
- Gauquelin, T.; Michon, G.; Joffre, R.; Duponnois, R.; Génin, D.; Fady, B.; Dagher-Kharrat, M.B.; Derridj, A.; Slimani, S.; Badri, W. Mediterranean forests, land use and climate change: A social-ecological perspective. Reg. Environ. Chang. 2018, 18, 623–636. [Google Scholar] [CrossRef]
- Cruz-Alonso, V.; Ruiz-Benito, P.; Villar-Salvador, P.; Rey-Benayas, J.M. Long-term recovery of multifunctionality in Mediterranean forests depends on restoration strategy and forest type. J. Appl. Ecol. 2019, 56, 745–757. [Google Scholar] [CrossRef]
- Himrane, H.; Camarero, J.J.; Gil-Pelegrín, E. Morphological and ecophysiological variation of the hybrid oak Quercus subpyrenaica (Q. faginea× Q. pubescens). Trees 2004, 18, 566–575. [Google Scholar]
- Wu, Z.-Y. Flora of China: Cycadaceae Through Fagaceae; Scientific Pubns: Beijing, China, 1999; Volume 4. [Google Scholar]
- Xu, X.; Dimitrov, D.; Shrestha, N.; Rahbek, C.; Wang, Z. A consistent species richness–climate relationship for oaks across the Northern Hemisphere. Glob. Ecol. Biogeogr. 2019, 28, 1051–1066. [Google Scholar] [CrossRef]



| Species Names | TSS | ROC | KAPPA | Sensitivity | Specificity | Number of Species Records |
|---|---|---|---|---|---|---|
| Quercus ×cerrioides ** | 1.00 | 1.00 | 0.97 | 100.0 | 99.9 | 18 |
| Quercus canariensis * | 0.98 | 1.00 | 0.78 | 100.0 | 97.5 | 96 |
| Quercus orocantabrica ** | 0.97 | 1.00 | 0.89 | 100.0 | 97.2 | 33 |
| Quercus estremadurensis * | 0.96 | 0.99 | 0.66 | 100.0 | 95.8 | 46 |
| Quercus lusitanica * | 0.93 | 0.99 | 0.72 | 97.6 | 95.0 | 212 |
| Quercus marianica ** | 0.92 | 0.99 | 0.75 | 100.0 | 92.4 | 70 |
| Quercus pubescens | 0.88 | 0.98 | 0.73 | 97.5 | 90.9 | 473 |
| Quercus subpyrenaica ** | 0.88 | 0.98 | 0.76 | 96.8 | 90.6 | 375 |
| Quercus broteroi | 0.86 | 0.98 | 0.70 | 93.5 | 92.4 | 306 |
| Quercus robur | 0.83 | 0.97 | 0.64 | 96.2 | 86.8 | 901 |
| Quercus petraea | 0.81 | 0.97 | 0.68 | 94.0 | 87.1 | 753 |
| Quercus faginea | 0.71 | 0.93 | 0.56 | 83.7 | 86.6 | 301 |
| Quercus pyrenaica | 0.66 | 0.91 | 0.56 | 86.7 | 79.4 | 3282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Viçosa, C.; Arenas-Castro, S.; Marcos, B.; Honrado, J.; García, C.; Vázquez, F.M.; Almeida, R.; Gonçalves, J. Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.). ISPRS Int. J. Geo-Inf. 2020, 9, 735. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi9120735
Vila-Viçosa C, Arenas-Castro S, Marcos B, Honrado J, García C, Vázquez FM, Almeida R, Gonçalves J. Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.). ISPRS International Journal of Geo-Information. 2020; 9(12):735. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi9120735
Chicago/Turabian StyleVila-Viçosa, Carlos, Salvador Arenas-Castro, Bruno Marcos, João Honrado, Cristina García, Francisco M. Vázquez, Rubim Almeida, and João Gonçalves. 2020. "Combining Satellite Remote Sensing and Climate Data in Species Distribution Models to Improve the Conservation of Iberian White Oaks (Quercus L.)" ISPRS International Journal of Geo-Information 9, no. 12: 735. https://0-doi-org.brum.beds.ac.uk/10.3390/ijgi9120735








