Vitis vinifera L. Pruning Waste for Bud-Preparations as Source of Phenolic Compounds–Traditional and Innovative Extraction Techniques to Produce New Natural Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Polyphenolic Content and Antioxidant Capacity
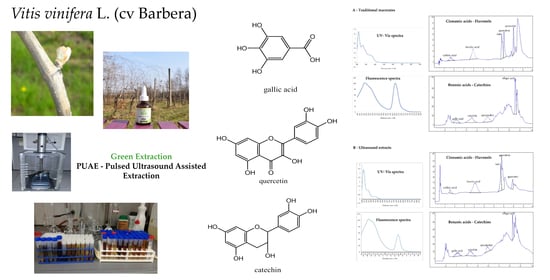
2.2. UV–Visible/Fluorescence Spectroscopy and HPLC Fingerprint
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Bud-Derivatives
3.2.1. Traditional Maceration
3.2.2. Pulsed Ultrasound-Assisted Extraction
3.3. Spectroscopic Analysis: Fluorescence and UV–Visible Fingerprint
3.3.1. UV–Vis Spectroscopy
3.3.2. Fluorescence Spectroscopy
3.4. HPLC Sample Preparation and Chromatographic Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calorio, C.; Donno, D.; Franchino, C.; Carabelli, V.; Marcantoni, A. Bud extracts from Salix caprea L. Inhibit voltage gated calcium channels and catecholamines secretion in mouse chromaffin cells. Phytomedicine 2017, 36, 168–175. [Google Scholar] [CrossRef]
- Turrini, F.; Vallarino, G.; Cisani, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Boggia, R.; Pittaluga, A.; Grilli, M. Use of an animal model to evaluate anxiolytic effects of dietary supplementation with Tilia tomentosa Moench bud extracts. Nutrients 2020, 12, 3328. [Google Scholar] [CrossRef]
- Allio, A.; Calorio, C.; Franchino, C.; Gavello, D.; Carbone, E.; Marcantoni, A. Bud extracts from Tilia tomentosa Moench inhibit hippocampal neuronal firing through gabaa and benzodiazepine receptors activation. J. Ethnopharmacol. 2015, 172, 288–296. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud extracts as new phytochemical source for herbal preparations: Quality control and standardization by analytical fingerprint. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: Rijeka, Croazia, 2015; Volume 1, pp. 187–218. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Carlen, C.; Ancay, A.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Analytical fingerprint and chemometrics as phytochemical composition control tools in food supplement analysis: Characterization of raspberry bud-preparations of different cultivars. J. Sci. Food Agric. 2016, 96, 3157–3168. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; ShriShriMal, N.; Das, D.K. Phytochemicals from plants to combat cardiovascular disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Turrini, F.; Boggia, R.; Guido, M.; Gamba, G.; Mellano, M.G.; Riondato, I.; Beccaro, G.L. Sustainable extraction and use of natural bioactive compounds from the waste management process of Castanea spp. bud-derivatives: The Finnover project. Sustainability 2020, 12, 10640. [Google Scholar] [CrossRef]
- Olivero, G.; Turrini, F.; Vergassola, M.; Boggia, R.; Zunin, P.; Donno, D.; Beccaro, G.L.; Grilli, M.; Pittaluga, A. The 3rs: Reduction and refinement through a multivariate statistical analysis approach in a behavioural study to unveil anxiolytic effects of natural extracts of Tilia tomentosa. Biomed. Sci. Eng. 2019, 3, s3. [Google Scholar] [CrossRef] [Green Version]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed ultrasound-assisted extraction as an alternative method to conventional maceration for the extraction of the polyphenolic fraction of Ribes nigrum buds: A new category of food supplements proposed by the Finnover project. Foods 2019, 8, 466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamba, G.; Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Randriamampionona, D.; Beccaro, G.L. Phytochemical characterization and bioactivity evaluation of autumn olive (Elaeagnus umbellata Thunb.) pseudodrupes as potential sources of health-promoting compounds. Appl. Sci. 2020, 10, 4354. [Google Scholar] [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Emerging species with nutraceutical properties: Bioactive compounds from Hovenia dulcis pseudofruits. Food Chem. 2020, 310, 125816. [Google Scholar] [CrossRef] [PubMed]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: Physico-chemical characterisation of bioactive components. Plant Food Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New findings in Prunus padus L. fruits as a source of natural compounds: Characterization of metabolite profiles and preliminary evaluation of antioxidant activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adebooye, O.C.; Alashi, A.M.; Aluko, R.E. A brief review on emerging trends in global polyphenol research. J. Food Biochem. 2018, 42, e12519. [Google Scholar] [CrossRef]
- D’Innocenzo, S.; Biagi, C.; Lanari, M. Obesity and the mediterranean diet: A review of evidence of the role and sustainability of the Mediterranean diet. Nutrients 2019, 11, 1306. [Google Scholar] [CrossRef] [Green Version]
- Militaru, A.V.; Simedrea, I.; Alexoi, I.; Peev, C.; Bernad, E.; Toma, C.-C. Plant extracts from meristematic tissues (foliar buds and shoots): Antioxidant and therapeutic action. Studia Univ. Vasile Goldis Arad. Ser. Stiintele Vietii 2010, 20, 45. [Google Scholar]
- Qsaib, S.; Mateus, N.; Ikbal, F.E.-z.; Rifai, L.A.; de Freitas, V.; Koussa, T. Direct identification and characterization of phenolic compounds from crude extracts of buds and internodes of grapevine (Vitis vinifera cv. Merlot). Nat. Prod. Commun. 2014, 9, 1569–1572. [Google Scholar] [CrossRef] [Green Version]
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Margină, D.; Olaru, O.T.; Ilie, M.; Grădinaru, D.; Guțu, C.; Voicu, S.; Dinischiotu, A.; Spandidos, D.A.; Tsatsakis, A.M. Assessment of the potential health benefits of certain total extracts from Vitis vinifera, Aesculus hyppocastanum and Curcuma longa. Exp. Ther. Med. 2015, 10, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Katalinić, V.; Generalić, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Bonvegna, L.; Bounous, G. Castanea spp. buds as a phytochemical source for herbal preparations: Botanical fingerprint for nutraceutical identification and functional food standardisation. J. Sci. Food Agric. 2014, 94, 2863–2873. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Cerutti, A.K.; Marconi, V.; Bounous, G. Botanicals in Ribes nigrum bud-preparations: An analytical fingerprinting to evaluate the bioactive contribution to total phytocomplex. Pharm. Biol. 2013, 51, 1282–1292. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.; Cerutti, A.; Beccaro, G. Biomolecules and natural medicine preparations: Analysis of new sources of bioactive compounds from Ribes and Rubus spp. Buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef]
- Hakimzadeh, N.; Parastar, H.; Fattahi, M. Combination of multivariate curve resolution and multivariate classification techniques for comprehensive high-performance liquid chromatography-diode array absorbance detection fingerprints analysis of Salvia reuterana extracts. J. Chromatogr. A 2014, 1326, 63–72. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Alves, R.C.; Oliveira, M.B.P.P. Herbal products containing Hibiscus sabdariffa L., Crataegus spp., and Panax spp.: Labeling and safety concerns. Food Res. Int. 2017, 100, 529–540. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Gamba, G.; Riondato, I.; Beccaro, G.L. Analytical strategies for fingerprinting of antioxidants, nutritional substances, and bioactive compounds in foodstuffs based on high performance liquid chromatography–mass spectrometry: An overview. Foods 2020, 9, 1734. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Kim, H.K.; Jeong, T.-S.; Lee, M.-K.; Park, Y.B.; Choi, M.-S.J.C.C.A. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Acta 2003, 327, 129–137. [Google Scholar] [CrossRef]
- Li, S.; Li, S.-K.; Gan, R.-Y.; Song, F.-L.; Kuang, L.; Li, H.-B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013, 51, 289–298. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [Green Version]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Babou, L.; Hadidi, L.; Grosso, C.; Zaidi, F.; Valentão, P.; Andrade, P.B. Study of phenolic composition and antioxidant activity of myrtle leaves and fruits as a function of maturation. Eur. Food Res. Technol. 2016, 242, 1447–1457. [Google Scholar] [CrossRef]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; Riondato, I.; De Biaggi, M.; Andriamaniraka, H.; Gamba, G.; Beccaro, G.L. Traditional and unconventional dried fruit snacks as a source of health-promoting compounds. Antioxidants 2019, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. agrobiodiversity conservation: Genotype influence on chemical and sensorial traits of cultivars grown on the same clonal rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef]
- Ordre National des Pharmaciens. Pharmacopée Française, Codex Medicamentarius Gallicus, Codex Français: Monographie, préparations Homéopathiques, 8th ed.; Ministère de la Santé Publique et de la Population, Ed.; Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM): Paris, France, 1965.
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef] [Green Version]
- Mok, D.K.W.; Chau, F.T. Chemical information of Chinese medicines: A challenge to chemist. Chemom. Intell. Lab. Syst. 2006, 82, 210–217. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Ruiz-Samblás, C.; Valverde-Som, L.; Pérez-Castaño, E.; González-Casado, A. Chromatographic fingerprinting: An innovative approach for food ‘identitation’ and food authentication—A tutorial. Anal. Chim. Acta 2016, 909, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Shi, X.; Zhang, Y.D.; Wang, Q. Quality evaluation of a herbal prescription through quantification of 40 components by HPLC-ESI-MS/MS. Phytochem. Anal. 2012, 23, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-Q.; Song, D.-F.; Li, R.-Q.; Yang, H.; Qi, L.-W.; Xin, G.-Z.; Wang, D.-Q.; Song, H.-P.; Chen, J.; Hao, H.; et al. Identification of effective combinatorial markers for quality standardization of herbal medicines. J. Chromatogr. A 2014, 1345, 78–85. [Google Scholar] [CrossRef] [PubMed]





| Caffeic Acid | Chlorogenic Acid | Coumaric Acid | Ferulic Acid | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ID | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | |||
| COMPANY_1 | C1 | 8.36 | 0.31 | a | n.d. | / | / | n.d. | / | / | 168.62 | 9.81 | a | |||
| COMPANY_2 | C2 | 8.22 | 0.17 | a | n.d. | / | / | n.d. | / | / | 190.01 | 5.51 | b | |||
| MACERATE | M | 7.86 | 0.19 | a | n.d. | / | / | n.d. | / | / | 234.44 | 9.51 | c | |||
| ULTRASOUND | US | 9.91 | 0.25 | b | n.d. | / | / | n.d. | / | / | 227.43 | 5.83 | c | |||
| Hyperoside | Isoquercitrin | Quercetin | Quercitrin | Rutin | ||||||||||||
| Sample | ID | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test |
| COMPANY_1 | C1 | n.d. | / | / | n.d. | / | / | 79.77 | 3.61 | a | 89.99 | 8.90 | a | 26.74 | 1.16 | a |
| COMPANY_2 | C2 | n.d. | / | / | n.d. | / | / | 91.23 | 3.74 | a | 105.95 | 5.86 | ab | 31.45 | 2.79 | a |
| MACERATE | M | n.d. | / | / | n.d. | / | / | 111.44 | 5.58 | b | 127.28 | 9.18 | b | 45.71 | 3.03 | b |
| ULTRASOUND | US | n.d. | / | / | n.d. | / | / | 106.86 | 8.71 | b | 356.10 | 9.85 | c | 95.86 | 4.12 | c |
| Ellagic Acid | Gallic acid | Catechin | Epicatechin | |||||||||||||
| Sample | ID | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | Mean Value | SD | Tukey Test | |||
| COMPANY_1 | C1 | 203.24 | 0.99 | a | 21.92 | 0.74 | a | 149.34 | 1.95 | ab | 42.37 | 2.59 | a | |||
| COMPANY_2 | C2 | 208.09 | 3.59 | a | 29.16 | 11.30 | a | 157.25 | 2.02 | b | 44.99 | 1.38 | ab | |||
| MACERATE | M | 222.35 | 1.03 | b | 29.42 | 0.69 | a | 145.13 | 5.15 | a | 67.67 | 2.85 | c | |||
| ULTRASOUND | US | 223.77 | 1.35 | b | 23.59 | 0.53 | a | 145.37 | 3.90 | a | 50.11 | 1.81 | b | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donno, D.; Turrini, F.; Boggia, R.; Guido, M.; Gamba, G.; Mellano, M.G.; Riondato, I.; Beccaro, G.L. Vitis vinifera L. Pruning Waste for Bud-Preparations as Source of Phenolic Compounds–Traditional and Innovative Extraction Techniques to Produce New Natural Products. Plants 2021, 10, 2233. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112233
Donno D, Turrini F, Boggia R, Guido M, Gamba G, Mellano MG, Riondato I, Beccaro GL. Vitis vinifera L. Pruning Waste for Bud-Preparations as Source of Phenolic Compounds–Traditional and Innovative Extraction Techniques to Produce New Natural Products. Plants. 2021; 10(11):2233. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112233
Chicago/Turabian StyleDonno, Dario, Federica Turrini, Raffaella Boggia, Maddalena Guido, Giovanni Gamba, Maria Gabriella Mellano, Isidoro Riondato, and Gabriele Loris Beccaro. 2021. "Vitis vinifera L. Pruning Waste for Bud-Preparations as Source of Phenolic Compounds–Traditional and Innovative Extraction Techniques to Produce New Natural Products" Plants 10, no. 11: 2233. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10112233