Anthropogenic Pressure on Tree Species Diversity, Composition, and Growth of Balanites aegyptiaca in Dinder Biosphere Reserve, Sudan
Abstract
:1. Introduction
2. Results
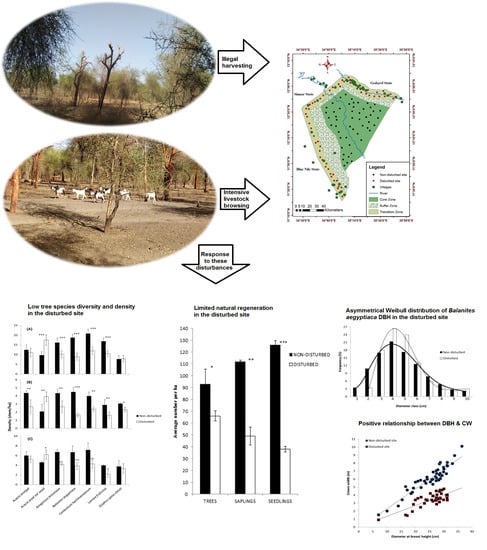
2.1. Low Tree Species Diversity and Density in Disturbed Sites
2.2. High Importance Value Index (IVI) and High Dominance in Disturbed Sites
2.3. Natural Regeneration and Growth form Distribution Are Highly Limited in Disturbed Sites
2.4. High Density and Crown Width of Balanites aegyptiaca in the Non-Disturbed Site
3. Discussion
3.1. Low Tree Species Diversity and Density in Disturbed Sites
3.2. High Importance Value Index (IVI) and Dominance in Disturbed Sites
3.3. Natural Regeneration and Growth form Distribution Limited in Disturbed Sites
3.4. High Density and Crown Width of Balanites aegyptiaca in the Non-Disturbed Sites
4. Materials and Methods
4.1. Study Area
4.2. Data Collection
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | N | Relative Abundance (%) | Relative Dominance (%) | Relative Frequency (%) | IVI |
|---|---|---|---|---|---|
| Acacia leata | 26 | 1.0 | 0.5 | 3.4 | 4.9 |
| Acacia mellifera | 56 | 2.2 | 1.0 | 3.9 | 7.1 |
| Acacia polyacantha | 35 | 1.4 | 0.8 | 2.0 | 4.1 |
| Acacia senegal | 144 | 7.8 | 6.2 | 7.8 | 21.8 |
| Acacia seyal var fistula | 65 | 2.6 | 1.7 | 3.9 | 8.2 |
| Acacia seyal var seyal | 514 | 27.8 | 37.1 | 14.5 | 79.4 |
| Adansonia digitata | 10 | 0.4 | 1.1 | 2.0 | 3.4 |
| Anogeissus leiocarpus | 97 | 3.8 | 3.9 | 2.9 | 10.7 |
| Balanites aegyptiaca | 187 | 10.1 | 13.6 | 12.7 | 36.4 |
| Combretum hartmannianum | 226 | 12.2 | 4.4 | 11.5 | 38.1 |
| Dichrostachys cinerea | 41 | 1.6 | 0.7 | 3.4 | 5.8 |
| Lannea fruticosa | 52 | 2.1 | 0.9 | 2.4 | 5.4 |
| Lonchocarpus laxiflorus | 171 | 9.3 | 3.5 | 6.6 | 19.4 |
| Sterculia setigera | 40 | 1.6 | 1.7 | 2.0 | 5.2 |
| Tamarindus indica | 12 | 0.6 | 0.8 | 2.0 | 3.4 |
| Terminalia brownii | 25 | 1.0 | 0.7 | 1.5 | 3.2 |
| Ziziphus spina-christi | 142 | 7.7 | 6.4 | 10.8 | 24.9 |
| Species | N | Relative Abundance (%) | Relative Dominance (%) | Relative Frequency (%) | IVI |
|---|---|---|---|---|---|
| Acacia polyacantha | 54 | 2.2 | 0.8 | 2.0 | 5.0 |
| Acacia senegal | 161 | 5.5 | 4.1 | 3.3 | 12.9 |
| Acacia seyal var fistula | 27 | 1.1 | 0.4 | 1.1 | 2.6 |
| Acacia seyal var seyal | 319 | 10.9 | 9.1 | 8.4 | 28.4 |
| Anogeissus leiocarpus | 182 | 7.4 | 8.8 | 5.1 | 21.3 |
| Balanites aegyptiaca | 358 | 16.7 | 17.8 | 13.5 | 48.0 |
| Boscia senegalensis | 31 | 1.3 | 0.5 | 3.6 | 5.3 |
| Boswellia papyrifera | 96 | 2.5 | 3.5 | 3.0 | 9.0 |
| Combretum aculeatum | 30 | 1.2 | 0.5 | 3.6 | 5.2 |
| Combretum ghasalense | 67 | 2.7 | 2.7 | 2.6 | 7.9 |
| Combretum glutinosum | 87 | 3.5 | 3.5 | 4.0 | 11.0 |
| Combretum hartmannianum | 321 | 11.0 | 14.8 | 6.4 | 36.2 |
| Commiphora africana | 32 | 1.3 | 1.9 | 2.3 | 5.5 |
| Dalbergia melanoxylon | 45 | 1.8 | 1.2 | 2.6 | 5.5 |
| Dichrostachys cinerea | 31 | 1.3 | 0.5 | 4.0 | 5.8 |
| Diospyros mespiliformis | 44 | 1.8 | 1.6 | 2.0 | 5.4 |
| Gardenia lutea | 36 | 1.5 | 0.6 | 3.6 | 5.7 |
| Hyphaena thebiaca | 49 | 2.0 | 2.9 | 2.6 | 7.4 |
| Lannea fruticosa | 184 | 4.5 | 4.4 | 5.6 | 14.5 |
| Lannea nigritana | 91 | 3.7 | 3.4 | 3.7 | 10.8 |
| Lannea schimperi | 36 | 1.5 | 1.9 | 1.7 | 5.1 |
| Maerua angolensis | 30 | 1.5 | 0.6 | 2.8 | 5.3 |
| Piliostigma reticulatum | 20 | 0.8 | 0.6 | 1.1 | 2.6 |
| Pseudocedreca kotschyi | 58 | 2.4 | 2.7 | 3.4 | 8.5 |
| Pterocarpus lucens | 50 | 2.0 | 3.1 | 2.0 | 7.1 |
| Sclerocarya birrea | 48 | 2.0 | 2.6 | 2.3 | 6.8 |
| Sterculia setigera | 84 | 3.4 | 5.0 | 4.0 | 12.3 |
| Stereospermum kunthianum | 16 | 0.7 | 0.9 | 1.1 | 2.6 |
| Strychnos innocua | 28 | 1.1 | 0.7 | 3.6 | 5.4 |
| Tamarindus indica | 19 | 0.8 | 1.0 | 0.9 | 2.6 |
| Terminalia brownii | 14 | 0.6 | 0.7 | 0.6 | 1.9 |
| Terminalia macroptera | 52 | 2.1 | 3.1 | 2.3 | 7.5 |
| Xeromphis nilotica | 29 | 1.5 | 0.4 | 3.7 | 5.6 |
| Ximenia americana | 41 | 1.7 | 0.9 | 2.8 | 5.3 |
| Ziziphus spina-christi | 154 | 5.3 | 3.0 | 5.1 | 13.4 |
| Species | N | Relative Abundance (%) | Relative Dominance (%) | Relative Frequency (%) | IVI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non | Dis | Non | Dis | Non | Dis | Non | Dis | Non | Dis | |
| Acacia senegal | 161 | 144 | 5.5 | 7.8 | 4.1 | 6.2 | 3.3 | 7.8 | 12.9 | 21.8 |
| Acacia seyal var seyal | 319 | 514 | 10.9 | 27.8 | 9.1 | 37.1 | 8.4 | 14.5 | 28.4 | 79.4 |
| Anogeissus leiocarpus | 182 | 97 | 7.4 | 3.8 | 8.8 | 3.9 | 5.1 | 2.9 | 21.3 | 10.7 |
| Balanites aegyptiaca | 358 | 187 | 16.7 | 10.1 | 17.8 | 13.6 | 13.5 | 12.7 | 48.0 | 36.4 |
| Combretum hartmannianum | 321 | 226 | 11.0 | 15.3 | 14.8 | 17.4 | 6.4 | 12.6 | 36.2 | 45.3 |
| Lannea fruticosa | 184 | 52 | 4.5 | 2.1 | 4.4 | 0.9 | 5.6 | 2.4 | 14.5 | 5.4 |
| Ziziphus spina-christi | 154 | 142 | 5.3 | 7.7 | 3.0 | 6.4 | 5.1 | 10.8 | 13.4 | 24.9 |
References
- Assogbadjo, A.E.; Kakaï, R.L.G.; Sinsin, B.; Pelz, D. Structure of Anogeissus leiocarpa Guill., Perr. natural stands in relation to anthropogenic pressure within Wari-Maro Forest Reserve in Benin. Afr. J. Ecol. 2010, 48, 644–653. [Google Scholar]
- Maua, J.O.; Tsingalia, H.M.; Cheboiwo, J.; Odee, D. Population structure and regeneration status of woody species in a remnant tropical forest: A case study of South Nandi forest, Kenya. Glob. Ecol. Conserv. 2020, 21, e00820. [Google Scholar] [CrossRef]
- Singh, S.; Malik, Z.A.; Sharma, C.M. Tree species richness, diversity, and regeneration status in different oak (Quercus spp.) dominated forests of Garhwal Himalaya, India. J. Asia Pac. Biodivers. 2016, 9, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Treydte, A.C.; Heitkönig, I.M.A.; Prins, H.H.T.; Ludwig, F. Trees improve grass quality for herbivores in African savannas. Perspect. Plant. Ecol. Evol. Syst. 2007, 8, 197–205. [Google Scholar] [CrossRef]
- Asigbaase, M.; Sjogersten, S.; Lomax, H.B.; Dawoe, E. Tree diversity and its ecological importance value in organic and conventional cocoa agroforests in Ghana. PLoS ONE 2019, 14, e0210557. [Google Scholar] [CrossRef] [Green Version]
- Belsky, A.J. Influences of trees on savanna productivity: Tests of shade, nutrients, and tree-grass competition. Ecology 1994, 75, 922–932. [Google Scholar] [CrossRef]
- Mukul, S.A.; Rashid, A.Z.M.M.; Uddin, M.B.; Khan, N.A. Role of non-timber forest products in sustaining forest-based livelihoods and rural households’ resilience capacity in and around protected area: A Bangladesh study. J. Environ. Plan. Manag. 2016, 59, 628–642. [Google Scholar] [CrossRef] [Green Version]
- Powell, B.; Ickowitz, A.; McMullin, S.; Jamnadass, R.; Padoch, C.; Pinedo-Vasquez, M.; Sunderland, T. The role of forests, trees and wild biodiversity for nutrition-sensitive food systems and landscapes. In Proceedings of the Expert Background Paper for the International Conference on Nutrition, Rome, Italy, 19–21 November 2014; pp. 1–25. [Google Scholar]
- Pfeifer, M.; Burgess, N.D.; Swetnam, R.D.; Platts, P.J.; Willcock, S.; Marchant, R. Protected areas: Mixed success in conserving East Africa’s evergreen forests. PLoS ONE 2012, 7, e39337. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, O.P.; Tripathi, R.S. Community composition, structure and management of subtropical vegetation of forests in Meghalaya State, northeast India. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2010, 6, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Abdou, L.; Morou, B.; Abasse, T.; Mahamane, A. Analysis of the structure and diversity of Prosopis africana (G. et Perr.) Taub. Tree stands in the Southeastern Niger. J. Plant Stud. 2016, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Idrissa, B.; Soumana, I.; Issiaka, Y.; Karimou, A.; Mahamane, A.; Mahamane, S.; Weber, J. Trend and Structure of Populations of Balanites aegyptiaca in Parkland Agroforestsin Western Niger. Annu. Res. Rev. Biol. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Taha, M.E.; Rizig, H.A.; Elamin, H.M.A.; Eltahir, M.E.S.; Bekele, T. Role of Non-Wood Forest Products in Welfare of Beneficiary Stakeholders in Sheikan Locality, North Kordofan State, Sudan. Int. J. Agric. For. Fish. 2015, 3, 129–136. [Google Scholar]
- Wassie, H.M. Potentials and Challenges of Alatish and Dinder National Parks (Ethiopia, Sudan)—Implementing Transboundary Park Cooperation. Master’s Thesis, University of Klagenfurt, Klagenfurt, Austria, 2011. [Google Scholar]
- Endale, Y.; Derero, A.; Argaw, M.; Muthuri, C. Farmland tree species diversity and spatial distribution pattern in semi-arid East Shewa, Ethiopia. For. Trees Livelihoods 2017, 26, 199–214. [Google Scholar] [CrossRef]
- Sales-Baptista, E.; d’Abreu, M.C.; Ferraz-de-Oliveira, M.I. Overgrazing in the Montado? The need for monitoring grazing pressure at paddock scale. Agrofor. Syst. 2016, 90, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Yousif, R.A.; Mohammed, F.A. Trends of poaching, Livestock Trespassing, Fishing and Resource Collection from 1986–2010 in Dinder National Park, Sudan. J. Life Sci. Biomed. 2012, 2, 105–109. [Google Scholar]
- Mohammed, A.N.E.; Hashim, I.M. Illegal and patrolling activities in Dinder National Park from 1959–2010. J. Nat. Resour. Environ. Stud. 2015, 6456, 22–32. [Google Scholar]
- Ball, L.; Tzanopoulos, J. Livestock browsing affects the species composition and structure of cloud forest in the Dhofar Mountains of Oman. Appl. Veg. Sci. 2020, 23, 363–376. [Google Scholar] [CrossRef]
- Kochare, T.; Tamir, B.; Kechero, Y. Palatability and animal preferences of plants in small and fragmented land holdings: The case of Wolayta Zone, Southern Ethiopia. Agric. Res. Technol. Open Access J. 2018, 14, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Sanon, H.O.; Kaboré-Zoungrana, C.; Ledin, I. Behaviour of goats, sheep and cattle and their selection of browse species on natural pasture in a Sahelian area. Small Rumin. Res. 2007, 67, 64–74. [Google Scholar] [CrossRef]
- Ssegawa, P.; Kasenene, J.M. Medicinal plant diversity and uses in the Sango bay area, Southern Uganda. J. Ethnopharmacol. 2007, 113, 521–540. [Google Scholar] [CrossRef]
- Suleiman, M.S.; Wasonga, V.O.; Mbau, J.S.; Suleiman, A.; Elhadi, Y.A. Non-timber forest products and their contribution to households income around Falgore Game Reserve in Kano, Nigeria. Ecol. Process. 2017, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fox, T.R. Sustained productivity in intensively managed forest plantations. For. Ecol. Manag. 2000, 138, 187–202. [Google Scholar] [CrossRef]
- Gustafsson, L.; Baker, S.C.; Bauhus, J.; Beese, W.J.; Brodie, A.; Kouki, J.; Lindenmayer, D.B.; Lhmus, A.; Pastur, G.M.; Messier, C.; et al. Retention forestry to maintain multifunctional forests: A world perspective. Bioscience 2012, 62, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Cantarello, E.; Lovegrove, A.; Orozumbekov, A.; Birch, J.; Brouwers, N.; Newton, A.C. Human impacts on forest biodiversity in protected Walnut-fruit forests in Kyrgyzstan. J. Sustain. For. 2014, 33, 454–481. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.K.; Raghubanshi, A.S.; Singh, J.S. Effect of grazing and harvesting on diversity, recruitment and carbon accumulation of juvenile trees in tropical dry forests. For. Ecol. Manag. 2012, 284, 152–162. [Google Scholar] [CrossRef]
- Seth, M.K. Trees and their economic importance. Bot. Rev. 2004, 69, 321–376. [Google Scholar] [CrossRef]
- Sukhbaatar, G.; Baatarbileg, N.; Battulga, P.; Batsaikhan, G.; Khishigjargal, M.; Batchuluun, T.; Gradel, A. Which selective logging intensity is most suitable for the maintenance of soil properties and the promotion of natural regeneration in highly continental scots pine forests?—Results 19 years after harvest operations in Mongolia. Forests 2019, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Elfeel, A.; Warrag, I.; Musnal, A. Response of Balanites aegyptiaca (L.) Del. Seedlings from Varied Geographical Source to Imposed Drought Stress. Discov. Innov. 2007, 18, 319–325. [Google Scholar]
- Fadl, K.E.M. Balanites aegyptiaca (L.): A Multipurpose Fruit Tree in Savanna Zone of Western Sudan. Int. J. Environ. 2015, 4, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahim, M. Contribution of Non-wood Forest Products in Support of Livelihoods of Rural People Living in the Area South of Blue Nile State, Sudan. Int. J. Agric. For. Fish. 2015, 3, 189–194. [Google Scholar]
- Adam, Y.O.; Pretzsch, J.; Pettenella, D. Contribution of Non-Timber Forest Products livelihood strategies to rural development in drylands of Sudan: Potentials and failures. Agric. Syst. 2013, 117, 90–97. [Google Scholar] [CrossRef]
- Younis, A.; Younis, I.; Mustafa, H.F.; Ballal, M.A. Perception of the Local Community Towards Utilization and Role of Non-wood Forest Products in Bahr Alarab Locality, East. IOSR J. Agric. Vet. Sci. 2018, 11, 74–81. [Google Scholar]
- Tesfaye, A. Balanites (Balanites aegyptiaca) Del., Multipurpose Tree a Prospective Review. Int. J. Mod. Chem. Appl. Sci. 2015, 3, 189–194. [Google Scholar]
- Hassanin, K.M.A.; Mahmoud, M.O.; Hassan, H.M.; Abdel-Razik, A.R.H.; Aziz, L.N.; Rateb, M.E. Balanites aegyptiaca ameliorates insulin secretion and decreases pancreatic apoptosis in diabetic rats: Role of SAPK/JNK pathway. Biomed. Pharmacother. 2018, 102, 1084–1091. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Motaal, A.; El-Askary, H.; Crockett, S.; Kunert, O.; Sakr, B.; Shaker, S.; Grigore, A.; Albulescu, R.; Bauer, R. Aldose reductase inhibition of a saponin-rich fraction and new furostanol saponin derivatives from Balanites aegyptiaca. Phytomedicine 2015, 22, 829–836. [Google Scholar] [CrossRef]
- Abou-Khalil, N.S.; Abou-Elhamd, A.S.; Wasfy, S.I.A.; El Mileegy, I.M.H.; Hamed, M.Y.; Ageely, H.M. Antidiabetic and Antioxidant Impacts of Desert Date (Balanites aegyptiaca) and Parsley (Petroselinum sativum) Aqueous Extracts: Lessons from Experimental Rats. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chapagain, B.P.; Yehoshua, Y.; Wiesman, Z. Desert date (Balanites aegyptiaca) as an arid lands sustainable bioresource for biodiesel. Bioresour. Technol. 2009, 100, 1221–1226. [Google Scholar] [CrossRef]
- Kikoti, I.; Mligo, C. Impacts of livestock grazing on plant species composition in montane forests on the northern slope of Mount Kilimanjaro, Tanzania. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Luginbuhl, J.; Mueller, J.P.; Green, T.J.; Chamblee, S.D.; Glennon, M.H. Grazing and Browsing Behavior, Grazing Management, Forage Evaluation and Goat Performance: Strategies to Enhance Meat Goat Production in North Carolina. Agric. Life Sci. 2010, 1, 73–87. [Google Scholar]
- Müller, K.; Dickhoefer, U.; Lin, L.; Glindemann, T.; Wang, C.; Schönbach, P.; Wan, H.W.; Schiborra, A.; Tas, B.M.; Gierus, M.; et al. Impact of grazing intensity on herbage quality, feed intake and live weight gain of sheep grazing on the steppe of Inner Mongolia. J. Agric. Sci. 2014, 152, 153–165. [Google Scholar] [CrossRef]
- Weldemariam, C.E.; Jakisa, S.E.; Ahebwe, A.D. Implication of forest zonation on tree species composition, diversity and structure in Mabira Forest, Uganda. Environ. Earth Ecol. 2017, 1, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Kacholi, D.S. Analysis of Structure and Diversity of the Kilengwe Forest in the Morogoro Region, Tanzania. Int. J. Biodivers. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bohn, F.J.; Huth, A. The importance of forest structure to biodiversity-productivity relationships. R. Soc. Open Sci. 2017, 4, 160521. [Google Scholar] [CrossRef] [Green Version]
- Hofhansl, F.; Chacón-Madrigal, E.; Fuchslueger, L.; Jenking, D.; Morera-Beita, A.; Plutzar, C.; Silla, F.; Andersen, K.M.; Buchs, D.M.; Dullinger, S.; et al. Climatic and edaphic controls over tropical forest diversity and vegetation carbon storage. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fakhry, A.M.; Khazzan, M.M.; Aljedaani, G.S. Impact of disturbance on species diversity and composition of Cyperus conglomeratus plant community in southern Jeddah, Saudi. J. King Saud Univ. Sci. 2020, 32, 600–605. [Google Scholar] [CrossRef]
- Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of disturbance on understory vegetation across slovenian forest ecosystems. Forests 2019, 10, 1048. [Google Scholar] [CrossRef] [Green Version]
- Solar, R.R.d.C.; Barlow, J.; Andersen, A.N.; Schoereder, J.H.; Berenguer, E.; Ferreira, J.N.; Gardner, T.A. Biodiversity consequences of land-use change and forest disturbance in the Amazon: A multi-scale assessment using ant communities. Biol. Conserv. 2016, 197, 98–107. [Google Scholar] [CrossRef]
- Ogunwusi, A.A.; Onwualu, A.; Ogunsanwo, O. Comparative analysis of wood properties of Afzelia africana and Anogeissus leiocarpus Growing in Nigeria. Chem. Mater. Res. 2013, 3, 8–13. [Google Scholar]
- Bello, A.A.; Jimoh, A.A. Some physical and mechanical properties of African birch (Anogeissus leiocarpus) timber. J. Appl. Sci. Environ. Manag. 2018, 22, 79. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, Y.; Abdu, K.; Maigar, A.K. Efficacy of Anogeissus leiocarpus (DC.) as potential therapeutic agent against Trypanosomiasis diseases: A review. Int. J. Heal. Pharm. Res. 2017, 3, 1–9. [Google Scholar]
- Mann, A.; Yusuf, A.; Daniyan, S. TLC analysis and bioactivity screening of the stem bark extract of Anogeissus leiocarpus against multi-resistant Staphylococcus aureus and quantification of its phytoconstituents. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 187–203. [Google Scholar]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Eltayb, M.T.A.; Magid, T.D.A. Effect of Felling Period and Types on Acacia seyal (Del) Ability to Generate by Sprouts in Rawashda Forest, Gedarif State, Sudan. J. For. Prod. Ind. 2013, 2, 13–20. [Google Scholar]
- Neelo, J.; Teketay, D.; Kashe, K.; Masamba, W. Stand structure, diversity and regeneration status of woody species in open and exclosed dry woodland sites around Molapo farming areas of the Okavango Delta, Northeastern Botswana. Open J. For. 2015, 5, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Kimaro, J.; Lulandala, L. Human influences on tree diversity and composition of a coastal forest ecosystem: The case of Ngumburuni forest reserve, Rufiji, Tanzania. Int. J. For. Res. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Gebrehiwot, K.; Hundera, K. Species composition, plant community structure and natural regeneration status of Belete moist evergreen montane forest, Oromia regional state, Southwestern Ethiopia. Momona Ethiop. J. Sci. 2014, 6, 97. [Google Scholar] [CrossRef]
- Chen, J.; Tang, H. Effect of grazing exclusion on vegetation characteristics and soil organic carbon of Leymus chinensis grassland in northern China. Sustainability 2016, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Osman, E.M.H.; Idris, E.Z.A. Species Dynamics and Potential Disturbances in El Nour Natural Forest Reserve, Sudan. J. For. Prod. Ind. 2012, 1, 10–20. [Google Scholar]
- Ibrahim, E.; Hassan, T. Factors Affecting Natural Regeneration and Distribution of Trees Species in El-Nour Natural Forest Reserve. J. Nat. Resour. Environ. Stud. 2015, 6456, 16–21. [Google Scholar]
- Romijn, E.; Lantican, C.B.; Herold, M.; Lindquist, E.; Ochieng, R.; Wijaya, A.; Murdiyarso, D.; Verchot, L. Assessing change in national forest monitoring capacities of 99 tropical countries. For. Ecol. Manag. 2015, 352, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Yousif, R.A. Abundance, Mayas preference and distribution of Birds in Dinder national park, Sudan. World’s Vet. J. 2012, 2, 27–32. [Google Scholar]
- Saaid, A.A.; Mohammed, A.E.; Sara, A.M.; Samia, H.A. Strobiloestrus clarkii in Redunca redunca at Dinder National Park. Asian J. Appl. Sci. 2019, 12, 7–14. [Google Scholar]
- Wang, S.; Fan, J.; Li, Y.; Huang, L. Effects of grazing exclusion on biomass growth and species diversity among various grassland types of the Tibetan Plateau. Sustainability 2019, 11, 1705. [Google Scholar] [CrossRef] [Green Version]
- Dufour-Dror, J.-M. Influence of cattle grazing on the density of oak seedlings and saplings in a Tabor oak forest in Israel. Acta Oecolo. 2007, 31, 223–228. [Google Scholar] [CrossRef]
- Lopez-Sanchez, A.; Sdhroeder, J.; Roig, S.; Sobral, M.; Dirzo, R. Effects of cattle management on oak regeneration in northern Californian Mediterranean oak woodlands. PLoS ONE 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Hernández, M.P.G.; Silva-Pando, F.J. Grazing effects of ungulates in a Galician oak forest (northwest Spain). For. Ecol. Manag. 1996, 88, 65–70. [Google Scholar] [CrossRef]
- McEvoy, P.M.; McAdam, J.H.; Mosquera-Losada, M.R.; Rigueiro-Rodríguez, A. Tree regeneration and sapling damage of pedunculate oak Quercus robur in a grazed forest in Galicia, NW Spain: A comparison of continuous and rotational grazing systems. Agrofor. Syst. 2006, 66, 85–92. [Google Scholar] [CrossRef]
- Thom, D.; Seidl, R. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biol. Rev. Camb. Philos. Soc. 2016, 91, 760–781. [Google Scholar] [CrossRef]
- Mwakosya, J.; Mligo, C. The impacts of anthropogenic activities on the vegetation communities and structure in the western part of Rungwe forest reserve, Tanzania. Tanzania J. Sci. 2014, 40, 60–78. [Google Scholar]
- Carmona, C.P.; Azcárate, F.M.; Oteros-Rozas, E.; González, J.A.; Peco, B. Assessing the effects of seasonal grazing on holm oak regeneration: Implications for the conservation of Mediterranean dehesas. Biol. Conserv. 2013, 159, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Mahgoub, A.A.M. Changes in Vegetation Cover and Impacts of Human Population in Dinder National Park, Sudan During the Period from 1972 to 2013; Sudan Academy of Science (SAS): Khartoum, Sudan, 2014. [Google Scholar]
- Okia, C.A. Balanites Aegyptiaca: A Resource for Improving Nutrition and Income of Dryland Communities in Uganda; Bangor University: Bangor, Wales, UK, 2013; Volume 53. [Google Scholar]
- Ligate, E.J.; Wu, C.; Chen, C. Investigation of tropical coastal forest regeneration after farming and livestock grazing exclusion. J. For. Res. 2019, 30, 1873–1884. [Google Scholar] [CrossRef] [Green Version]
- Beche, D.; Gebeyehu, G.; Feyisa, K. Indigenous utilization and management of useful plants in and around Awash National Park, Ethiopia. J. Plant Biol. Soil Health 2016, 3, 1–12. [Google Scholar]
- Li, S.; Yu, F.; Werger, M.J.A.; Dong, M.; Ramula, S.; Zuidema, P.A. Understanding the effects of a new grazing policy: The impact of seasonal grazing on shrub demography in the Inner Mongolian steppe. J. Appl. Ecol. 2013, 50, 1377–1386. [Google Scholar] [CrossRef]
- Zamora, R.; Gómez, J.M.; Hódar, J.A.; Castro, J.; García, D. Effect of browsing by ungulates on sapling growth of Scots pine in a mediterranean environment: Consequences for forest regeneration. For. Ecol. Manag. 2001, 144, 33–42. [Google Scholar] [CrossRef]
- Sher, H.; Ahmed, A.; Eleyemeni, M.; Fazl-i-Hadi, S.; Sher, H. Impact of the Nomadic Grazing on Medicinal Plants Diversity in Miandam, Swat-Pakistan. Am. J. Sustain. Agric. 2010, 4, 152–159. [Google Scholar]
- Krzic, M.; Newman, R.F.; Trethewey, C.; Bulmer, C.E.; Chapman, B.K. Cattle grazing effects on plant species composition and soil compaction on rehabilitated forest landings in central interior British Columbia. J. Soil Water Conserv. 2006, 61, 137–144. [Google Scholar]
- Riginos, C.; Young, T.P. Positive and negative effects of grass, cattle, and wild herbivores on Acacia saplings in an East African savanna. Oecologia 2007, 153, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Riginos, C.; Grace, J.B.; Augustine, D.J.; Young, T.P. Local versus landscape-scale effects of savanna trees on grasses. J. Ecol. 2009, 97, 1337–1345. [Google Scholar] [CrossRef]
- Hassaballah, K.E.A. Land Degradation in the Dinder and Rahad Basins: Interactions between Hydrology, Morphology and Ecohydrology in the Dinder Nationalpark, Sudan. Ph.D. Thesis, Delft University of Technology, Delft, The Netherland, 2020. [Google Scholar]
- Hassaballah, K.; Mohamed, Y.A.; Uhlenbrook, S. The Mayas wetlands of the Dinder and Rahad: Tributaries of the Blue Nile Basin (Sudan). In The Wetland Book; Springer: Dordrecht, The Netherland, 2016; pp. 1–13. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Pantera, Α.; Fotiadis, G.; Papaspyropoulos, K.; Mantzanas, K.; Papanastasis, V.P. Effects of grazing and understorey clearing on regeneration of a valonia oak silvopastoral system in Western Greece. CEST 2017, 15, 1–4. [Google Scholar]
- Ibrahim, E.; Osman, E.; Idris, E.; Yousif, T. Linear and Non-Linear Regression Equations for Estimating the Crown Diameter of Three Sudanese Edible Trees. J. For. Prod. Ind. 2015, 4, 44–52. [Google Scholar]
- Ibrahim, E.; Osman, E. Diameter at Breast Height-Crown Width Prediction Models for Anogeissus Leiocarpus (DC.) Guill & Perr and Combretum Hartmannianum Schweinf. J. For. Prod. Ind. 2014, 3, 191–197. [Google Scholar]
- Ibrahim, E.; Osman, E.; Idris, E. Modelling the Relationship between Crown width and Diameter at Breast Height for Naturally grown Terminalia tree species. J. Nat. Resour. Environ. Stud. 2014, 6456, 42–49. [Google Scholar]
- Bekele, A. Useful Trees and Shrubs of Ethiopia: Identification, Propagation and Management in 17 Agro-Ecological Zones; Tengnas, B., Kelbesa, E., Demissew, S., Maundu, P., Eds.; World Agroforestry Centre: East Africa Region, Nairobi Kenya, 2007; ISBN 9966896031. [Google Scholar]
- Hernández, A.S.M.; González, M.F.A.; Martínez, V.A.; Posadas, M.D.H.; Aldrete, A.; Díaz, C.E. Structure, richness and diversity of tree species in a tropical deciduous forest of Morelos. Rev. Mex. Ciencias For. 2018, 9, 131–156. [Google Scholar]








| Parameter | Non-Disturbed Site | Disturbed Site | T | p |
|---|---|---|---|---|
| Mean (±SE) | Mean (±SE) | |||
| Tree DBH (cm) | 32.8 ± 0.7 | 27.5 ± 0.4 | 2.1 | 0.038 |
| Tree height (m) | 12.5 ± 0.2 | 9.6 ± 0.1 | 3.6 | 0.001 |
| Tree crown width (m) | 7.7 ± 0.2 | 3.3 ± 0.1 | 3.2 | 0.002 |
| Tree basal area (m2 ha−1) | 0.07 ± 0.03 | 0.06 ± 0.02 | 2.1 | 0.034 |
| Tree volume (m3) | 0.39 ± 0.02 | 0.27 ± 0.10 | 2.9 | 0.004 |
| Tree density (stem ha−1) | 18.7 ± 0.4 | 8.9 ± 1.2 | 2.6 | 0.018 |
| Basal area contribution (%) | 9.5 ± 0.3 | 23.1 ± 1.3 | 30.8 | <0.001 |
| Blackman index | 0.52 ± 0.30 | 4.02 ± 0.40 | 39.3 | <0.001 |
| Parameter | Equation | Reference |
|---|---|---|
| Tree basal area (g) | g = (π ÷ 4) × DBHi2 | [61] |
| Stand basal area (G) | G = (n ÷ 4 s) × ∑DBHi2 | [12] |
| Stand density (N) | N = n ÷ s | [1] |
| Species abundance (A) | A = Total number of trees for a species ÷ Area sampled | [61] |
| Relative abundance (RA) | RA = (Abundance of a species ÷ Total abundance for all) × 100 | [12] |
| Species dominance (D) | D = Total basal area of a species ÷ area sampled | [61] |
| Relative dominance (RD) | RD = (Coverage of a species ÷ Total coverage for all) × 100 | [12] |
| Species frequency (F) | F = Occurrence or absence of species in a sample plot | [60] |
| Relative frequency (RF) | RF = (Frequency of a species ÷ Total frequency for all) × 100 | [61] |
| Importance Value Index | IVI = RA + RD + RF | [12] |
| Blackman Index (IB) | IB = ÷ | [1] |
| Basal area contribution | Cs = (GB ÷ G) × 100 | [1] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, E.M.I.; H., E.A.M.; Ndakidemi, P.A.; Treydte, A.C. Anthropogenic Pressure on Tree Species Diversity, Composition, and Growth of Balanites aegyptiaca in Dinder Biosphere Reserve, Sudan. Plants 2021, 10, 483. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030483
Mohammed EMI, H. EAM, Ndakidemi PA, Treydte AC. Anthropogenic Pressure on Tree Species Diversity, Composition, and Growth of Balanites aegyptiaca in Dinder Biosphere Reserve, Sudan. Plants. 2021; 10(3):483. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030483
Chicago/Turabian StyleMohammed, Elmugheira M. I., Elhag A. M. H., Patrick A. Ndakidemi, and Anna C. Treydte. 2021. "Anthropogenic Pressure on Tree Species Diversity, Composition, and Growth of Balanites aegyptiaca in Dinder Biosphere Reserve, Sudan" Plants 10, no. 3: 483. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10030483