Triterpenoids from Vitellaria paradoxa Stem Barks Reduce Nitrite Levels in LPS-Stimulated Macrophages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Characterization of Triterpenoids
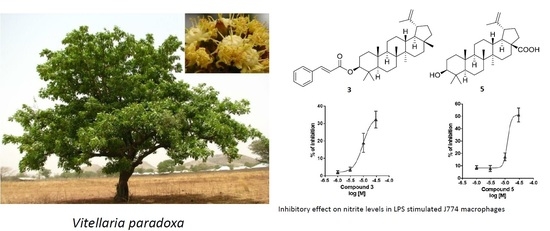
2.2. Biological Activity of Triterpenoids
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Chromatographic Purification
3.3.1. Ursaldehyde Cinnamate (7)
3.3.2. 11-Hydroxy-β-Amyrin Cinnamate (10)
3.4. Pharmacological Evaluation
3.4.1. Cell Culture
3.4.2. Nitrite Measurement and Pharmacological Treatment In Vitro
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadembega, P.; Boussim, J.I.; Nikiema, J.B.; Poli, F.; Antognoni, F. Medicinal plants in Baskoure, Kourittenga province, Burkina Faso: An ethnobotanical study. J. Ethnopharmacol. 2011, 133, 378–395. [Google Scholar] [CrossRef]
- Verma, N.; Chakrabarti, R.; Das, R.H.; Gautam, H.K. Anti-inflammatory effects of shea butter through inhibition of iNOS, Cox2 and cytokines via the Nf-Kb Pathway in LPS-activated J774 macrophage cells. J. Complement. Integr. Med. 2012, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Foyet, H.S.; Tsala, D.E.; Zogo Essono Bodo, J.C.; Carine, A.N.; Heroyne, L.T.; Oben, E.K. Anti-inflammatory and anti-arthritic activity of a methanol extract from Vitellaria paradoxa stem bark. Pharmacognosy Res. 2015, 7, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyong, K.O.; Foyet, H.S.; Baïrysa, G.; Folefoc, G.N.; Asongalem, E.A.; Lagojda, A.; Lamshöft, M. A new ursane triterpenoic acid and other potential anti-inflammatory and anti-arthritic constituents from EtOAc extracts of Vitellaria paradoxa stem bark. J. Ethnopharmacol. 2015, 174, 277–286. [Google Scholar] [CrossRef]
- Akihisa, T.; Kojima, N.; Kikuchi, T.; Yasukawa, K.; Tokuda, H.; Masters, E.T.; Manosroi, A.; Manosroi, J. Anti-inflammatory and chemopreventive effects of triterpene cinnamates and acetates from shea fat. J. Oleo Sci. 2010, 59, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Sudirman, S.; Chen, C.-K.; Long, B.-T.; Chang, H.-W.; Tsou, D.; Kong, Z.-L. Vitellaria paradoxa Nut Triterpene-Rich Extract Ameliorates Symptoms of Inflammation on Post-Traumatic Osteoarthritis in Obese Rats. J. Pain Res. 2020, 13, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filip, S.; Djarmati, Z.; Lisichkov, K.; Csanadi, J.; Jankov, R.M. Isolation and characterization of Maclura (Maclura pomifera) extracts obtained by supercritical fluid extraction. Ind. Crops. Prod. 2015, 76, 995–1000. [Google Scholar] [CrossRef]
- Manzano, P.I.; Miranda, M.; Abreu-Payrol, J.; Silva, M.; Sterner, O.; Peralta, E.L. Pentacyclic triterpenoids with antimicrobial activity from the leaves of Vernonanthura patens (Asteraceae) Emir. J. Food Agric. 2013, 25, 539–543. [Google Scholar]
- Lee, H.-S.; Kim, E.-N.; Jeong, G.-S. Lupenone Protects Neuroblastoma SH-SY5y Cells Against Methamphetamine-Induced Apoptotic Cell Death via PI3K/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1617. [Google Scholar] [CrossRef] [Green Version]
- Eyong, K.O.; Bairy, G.; Eno, A.A.; Taube, J.; Hull, K.G.; Folefoc, G.N.; Foyet, H.S.; Romo, D. Triterpenoids from the stem bark of Vitellaria paradoxa (Sapotaceae) and derived esters exhibit cytotoxicity against a breast cancer cell line. Med. Chem. Res. 2018, 27, 268–277. [Google Scholar] [CrossRef]
- Dais, P.; Plessel, R.; Williamson, K.; Hatzakis, E. Complete 1H and 13C NMR assignment and 31P NMR determination of pentacyclic triterpenic acids. Anal. Methods 2017, 9, 949–958. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chiang, Y.M. Six new ursane- and oleanane-type triterpenes from the aerial roots of Ficus macrocarpa. Chem. Pharm. Bull. 2000, 48, 593–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hota, R.K.; Bapuji, M. Triterpenoids from the resin of Shorea robusta. Phytochemistry 1994, 35, 1073–1074. [Google Scholar] [CrossRef]
- Ikuta, A.; Morikawa, A. Triterpenes from Stautonia hexaphylla call tissues. J. Nat. Prod. 1992, 55, 1230–1233. [Google Scholar] [CrossRef]
- Akihisa, T.; Kojima, N.; Katoh, N.; Kikuchi, T.; Fukatsu, M.; Shimizu, N.; Masters, E.T. Triacylglycerol and triterpene ester composition of shea nuts from seven African countries. J. Oleo Sci. 2011, 60, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kurita, M.; Shinozaki, T.; Ukiya, M.; Yasukawa, K.; Shimizu, N.; Tokuda, H.; Masters, E.T.; Akihisa, M.; Akihisa, T. Triterpene glycosides and other polar constituents of shea (Vitellaria paradoxa) kernels and their bioactivities. Phytochemistry 2014, 108, 157–170. [Google Scholar] [CrossRef]
- Catteau, L.; Schioppa, L.; Beaufay, C.; Girardi, C.; Hérent, M.-F.; Frédérich, M.; Quetin-Leclercq, J. Antiprotozoal activities of Triterpenic Acids and Ester Derivatives Isolated from the Leaves of Vitellaria paradoxa. Planta Med. 2020. [Google Scholar] [CrossRef]
- Tapondjou, L.A.; Nyaa, L.B.T.; Tane, P.; Ricciutelli, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Ponou, B.K.; Barboni, L. Cytotoxic and antioxidant triterpene saponins from Butyrospermum parkii (Sapotaceae). Carbohydr. Res. 2011, 346, 2699–2704. [Google Scholar] [CrossRef]
- Cheras, P.A.; Myers, S.P.; Paul-Brent, P.-A.; Outerbridge, K.H.; Nielsen, G.V.L. Randomized Double-Blind Placebo-Controlled Trial on the Potential Modes of Action of SheaFlex70TM in Osteoarthritis. Phytother. Res. 2010, 24, 1126–1131. [Google Scholar] [CrossRef]
- Chicca, A.; Marazzi, J.; Gertsch, J. The antinociceptive triterpene β-amyrin inhibits 2-arachidonoylglycerol (2-AG) hydrolysis without directly targeting cannabinoid receptors. Br. J. Pharmacol. 2012, 167, 1596–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, B.; Pagano, E.; Orlando, P.; Capasso, R.; Cascio, M.G.; Pertwee, R.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. Pure Δ9-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Δ9-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol. Res. 2016, 113, 199–208. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirignano, C.; Nadembega, P.; Poli, F.; Romano, B.; Lucariello, G.; Rigano, D.; Taglialatela-Scafati, O. Triterpenoids from Vitellaria paradoxa Stem Barks Reduce Nitrite Levels in LPS-Stimulated Macrophages. Plants 2021, 10, 1006. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10051006
Sirignano C, Nadembega P, Poli F, Romano B, Lucariello G, Rigano D, Taglialatela-Scafati O. Triterpenoids from Vitellaria paradoxa Stem Barks Reduce Nitrite Levels in LPS-Stimulated Macrophages. Plants. 2021; 10(5):1006. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10051006
Chicago/Turabian StyleSirignano, Carmina, Pascal Nadembega, Ferruccio Poli, Barbara Romano, Giuseppe Lucariello, Daniela Rigano, and Orazio Taglialatela-Scafati. 2021. "Triterpenoids from Vitellaria paradoxa Stem Barks Reduce Nitrite Levels in LPS-Stimulated Macrophages" Plants 10, no. 5: 1006. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10051006