Reclaiming to Brackish Wetlands in the Alberta Oil Sands: Comparison of Responses to Sodium Concentrations by Carex atherodes and Carex aquatilis
Abstract
:1. Introduction
- How do the structural attributes of C. atherodes respond to a series of Na+ concentration treatments that are present or expected at future in-pit reclamation sites in the Alberta oil sands region?
- Are different structural responses related to functional attributes of chlorophyll, photosynthesis, stomatal conductance, and/or transpiration rate?
- How do these responses compare to those of C. aquatilis?
2. Results
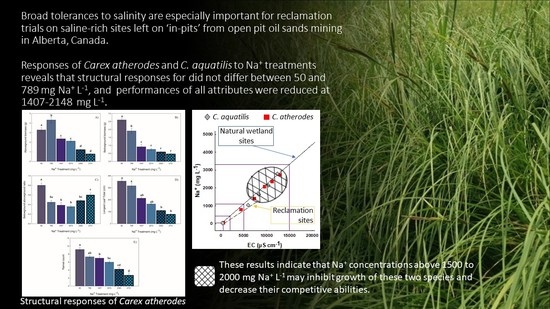
2.1. Structural Attributes
2.1.1. Biomass
2.1.2. Longest Leaf Length
2.1.3. Ramet Count
2.2. Chlorophyll Content
2.3. Functional Attributes
2.3.1. Photosynthetic Rate
2.3.2. Transpiration Rate
2.3.3. Stomatal Conductance
2.3.4. Concentration of Na in Aboveground and Belowground Biomass
2.3.5. Comparison to Carex aquatilis
3. Discussion
3.1. Variation in Sodium at Natural Sites and at Sandhill Wetland
3.2. Carex atherodes—Responses of Structural and Functional Attributes
3.3. Comparison to Carex Aquatilis
4. Materials and Methods
4.1. Experimental Design
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indigenous Environmental Network. 2010. Available online: https://www.ienearth.org/what-are-the-tar-sands/ (accessed on 20 July 2021).
- Government of Alberta. Oil Sands. Reclamation; Government of Alberta: Edmonton, AB, Canada, 2020. Available online: http://oilsands.alberta.ca/FactSheets/FactSheet-Reclamation-2015.pdf (accessed on 21 February 2020).
- Foote, L. Threshold considerations and wetland reclamation in Alberta’s mineable oil sands. Ecol. Soc. 2012, 17, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chalaturnyk, R.J.; Scott, D.J.; Ozum, B. Management of oil sands tailings. Pet. Sci. Technol. 2002, 20, 1025–1046. [Google Scholar] [CrossRef]
- Matthews, J.G.; Shaw, W.H.; MacKinnon, M.D.; Cuddy, R.G. Development of composite tailings technology at Syncrude. Int. J. Surf. Min. Reclam. Environ. 2002, 16, 24–39. [Google Scholar] [CrossRef]
- MacKinnon, M.D.; Matthews, J.G.; Shaw, W.H.; Cuddy, R.G. Water quality issues associated with composite tailings (CT) technology for managing oil sands tailings. Int. J. Surf. Min. Reclam. Environ. 2001, 15, 235–256. [Google Scholar] [CrossRef]
- Harris, M.L. Guideline for Wetland Establishment on Reclaimed Oil Sands Leases, 2nd ed.; Reclamation Working Group, Cumulative Environmental Management Association: Fort McMurray, AB, Canada, 2007; p. 117. [Google Scholar]
- Johnson, E.A.; Miyanishi, K. Creating new landscapes and ecosystems: The Alberta oil sands. Ann. N. Y. Acad. Sci. 2008, 1134, 120–145. [Google Scholar] [CrossRef]
- Wieder, R.K.; Vile, M.A.; Scott, K.D.; Albright, C.M.; McMillen, K.J.; Vitt, D.H.; Fenn, M.E. Differential effects of high atmospheric N and S deposition on bog plant/lichen tissue and porewater chemistry across the Athabasca Oil Sands Region. Environ. Sci. Technol. 2016, 50, 12630–12640. [Google Scholar] [CrossRef] [PubMed]
- Rooney, R.C.; Bayley, S.E.; Schindler, D.W. Oil sands mining and reclamation cause massive loss of peatland and stored carbon. Proc. Natl. Acad. Sci. USA 2012, 109, 4933–4937. [Google Scholar] [CrossRef] [Green Version]
- Vitt, D.H.; Chee, W.L. The relationships of vegetation to surface-water chemistry and peat chemistry in fens of Alberta, Canada. Vegetatio 1990, 89, 87–106. [Google Scholar] [CrossRef]
- Hartsock, J.A.; House, M.; Clark, M.G.; Vitt, D.H. A comparison of plant communities and water chemistry at Sandhill Wetland to natural Albertan peatlands and marshes. Ecol. Eng. 2021, 169, 106313. [Google Scholar] [CrossRef]
- Purdy, B.G.; MacDonald, S.E.; Lieffers, V.J. Naturally saline boreal communities as models for reclamation of saline oil sand tailings. Restor. Ecol. 2005, 13, 667–677. [Google Scholar] [CrossRef]
- Raab, D.; Bayley, S.E. A Carex species-dominated marsh community represents the best short-term target for reclaiming wet meadow habitat following oil sands mining in Alberta, Canada. Ecol. Eng. 2013, 54, 97–106. [Google Scholar] [CrossRef]
- Stewart, S.A.; Lemay, T.G. Inorganic Water Chemistry of Saline Fens in Northeastern Alberta (NTS 74D); ERCB/AGS Open File Report 2011–09; Energy Resource Conservation Board, Alberta Geological Survey: Edmonton, AB, Canada, 2011; p. 6. [Google Scholar]
- Trites, M.; Bayley, S.E. Vegetation communities in continental boreal wetlands along a salinity gradient: Implications for oil sands mining reclamation. Aquat. Bot. 2009, 91, 27–39. [Google Scholar] [CrossRef]
- Vitt, D.; Bhatti, J. Incorporating Ecological Theory into Restoration Planning. In Restoration and Reclamation of Boreal Ecosystems: Attaining Sustainable Development; Vitt, D.H., Bhatti, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 3–12. [Google Scholar]
- Wytrykush, C.; Vitt, D.H.; McKenna, G.; Vassov, R. Designing Landscapes to Support Peatland Development on Soft Tailings Deposits. In Restoration and Reclamation of Boreal Ecosystems: Attaining Sustainable Development; Vitt, D.H., Bhatti, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 161–178. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.C.; Mollard, F.P.O.; Foote, A.L. Do peat amendments to oil sands wet sediments affect Carex aquatilis biomass for reclamation success? J. Environ. Manag. 2014, 139, 154–163. [Google Scholar] [CrossRef]
- Pouliot, R.; Rochefort, L.; Graf, M.D. Impacts of oil sands process water on fen plants: Implications for plant selection in required reclamation projects. Environ. Pollut. 2012, 167, 132–137. [Google Scholar] [CrossRef]
- Hultén, E. Flora of Alaska and Neighboring Territories; Stanford University Press: Stanford, CA, USA, 1968; p. 1008. [Google Scholar]
- Jeffrey, W.W. Notes on plant occurrence along lower liard river, N.W.T. National Museum of Canada. Bulletin 1961, 171, 32–115. [Google Scholar]
- Johnson, D.; Kershaw, L.; MacKinnon, A.; Pojar, J. Plants of the Western Boreal Forest & Aspen Parkland; Lone Pine Publishing and the Canadian Forest Service: Edmonton, AB, Canada, 1995. [Google Scholar]
- Alberta Environment and Sustainable Resource Development (ESRD). Alberta Wetland Classification System; Water Policy Branch, Policy and Planning Division: Edmonton, AB, Canada, 2015.
- Stewart, R.E.; Kantrud, H.A. Vegetation of Prairie Potholes, North Dakota, in Relation to Quality of Water and Other Environmental Factors; Hydrology of Prairie Potholes in North Dakota; U.S. Geological Survey Professional Paper 585D; United States Government Printing Office: Washington, DC, USA, 1972; 35p. [CrossRef] [Green Version]
- Millar, J.B. Wetland classification in western Canada. In A Guide to Marshes and Shallow Open Water Wetlands in the Grasslands and Parklands of the Prairie Provinces; Report Series Number 37; Canadian Wildlife Service, Environment Canada: Ottawa, ON, Canada, 1976. [Google Scholar]
- Walker, B.H.; Coupland, R.T. Herbaceous wetland vegetation in the aspen grove and grassland regions of Saskatchewan. Can. J. Bot. 1970, 48, 696–711. [Google Scholar] [CrossRef]
- Zoltai, S.C.; Taylor, S.; Jeglum, J.K.; Mills, G.F.; Johnson, J.D. Wetlands of Boreal Canada. In National Wetlands Working Group, Wetlands of Canada, Ecological Land Classification Series No. 24; Sustainable Development Branch, Environment Canada: Ottawa, ON, Canada; Polyscience Publications, Inc.: Montreal, QC, Canada, 1988; Volume 24, pp. 97–154. [Google Scholar]
- Koropchak, S.; Vitt, D.H.; Bloise, R.; Wieder, R.K. Fundamental Paradigms, Foundation Species Selection, and Early Plant Responses to Peatland Initiation on Mineral Soils. In Restoration and Reclamation of Boreal Ecosystems: Attaining Sustainable Development; Vitt, D.H., Bhatti, J., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 76–100. [Google Scholar]
- Vitt, D.H.; House, M.; Hartsock, J.A. Final Report for the Ecology of Sandhill Fen 2016–2019; Syncrude Canada, Ltd.: Fort McMurray, AB, Canada, 2020. [Google Scholar]
- Vitt, D.H.; House, M.; Hartsock, J.A. Sandhill Fen, an initial trial for wetland species assembly on in-pit substrates: Lessons after three years. Botany 2016, 94, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Chee, W.L.; Vitt, D.H. The vegetation, surface-water chemistry and peat chemistry of moderate-rich fens in central Alberta, Canada. Wetlands 1989, 9, 227–261. [Google Scholar] [CrossRef]
- Nicholson, B.J. The wetlands of Elk Island National Park: Vegetation classification, water chemistry, and hydrotopographic relationships. Wetlands 1995, 15, 119–133. [Google Scholar] [CrossRef]
- Slack, N.G.; Vitt, D.H.; Horton, D.G. Vegetation gradients of minerotrophically rich fens in western Alberta. Can. J. Bot. Rev. Can. Bot. 1980, 58, 330–350. [Google Scholar] [CrossRef]
- Nicholson, B.J.; Vitt, D.H. Wetland development at Elk Island National Park, Alberta, Canada. J. Paleolimnol. 1994, 12, 19–34. [Google Scholar] [CrossRef]
- House, M.; Vitt, D.H.; Glaeser, L.; Hartsock, J. Changing chemical conditions and water levels at Sandhill Wetland, Alberta, Canada: Effects on plant distributions and abundance. J. Hydrol. Reg. Stud. 2021. under review. [Google Scholar]
- Vitt, D.H.; Glaeser, L.C.; House, M.; Kitchen, S.P. Structural and functional responses of Carex aquatilis to increasing sodium concentrations. Wetl. Ecol. Manag. 2020, 28, 753–763. [Google Scholar] [CrossRef]
- Koropchak, S.; Vitt, D.H. Survivorship and growth of Typha latifolia L. across a NaCl gradient: A greenhouse study. Int. J. Min. Reclam. Environ. 2013, 27, 143–150. [Google Scholar] [CrossRef]
- Glaeser, L.C.; Vitt, D.H.; Ebbs, S. Responses of the wetland grass, Beckmannia syzigachne, to salinity and soil wetness: Consequences for wetland reclamation in the oil sands area of Alberta, Canada. Ecol. Eng. 2016, 86, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Iseki, K.; Marubodee, R.; Ehara, H.; Tomooka, N. A rapid quantification method for tissue Na+ and K+ concentrations in salt-tolerant and susceptible accessions in Vigna vexillata (L.) A. Rich. Plant Prod. Sci. 2017, 20, 144–148. [Google Scholar] [CrossRef]
- SigmaPlot; Version 11; Systat Software, Inc.: San Jose, CA, USA, 2008.
- Subbarao, G.V.; Johansen, C.; Jana, M.K.; Rao, J.K. Effects of the sodium/calcium ratio in modifying salinity response of pigeonpea (Cajanus cajan). J. Plant Physiol. 1990, 136, 439–443. [Google Scholar] [CrossRef]



| Carex aquatilis Results | 40 mg Na+ L−1 | 1035 mg Na+ L−1 | 1650 mg Na+ L−1 | 2148 mg Na+ L−1 | 2792 mg Na+ L−1 |
|---|---|---|---|---|---|
| Aboveground biomass (g) | 2.07 ± 0.18 a | 1.49 ± 0.22 ab | 1.29 ± 0.13 ab | 0.96 ± 0.34 b | 0.78 ± 0.17 b |
| Belowground biomass (g) | 2.34 ± 0.23 a | 1.60 ± 0.27 ab | 1.35 ± 0.31 ab | 0.84 ± 0.39 b | 0.54 ± 0.09 b |
| Longest leaf length (cm) | 265.88 ± 35.68 a | 145.84 ± 15.64 ab | 146.38 ± 8.79 ab | 91.75 ± 23.84 b | 77.83 ± 11.47 b |
| Ramet count | 5.66 ± 1.54 a | 3.25 ± 0.55 a | 5.67 ± 0.67 a | 3.17 ± 0.98 a | 3.17 ± 0.83 a |
| Below:aboveground biomass | 1.15 ± 0.08 a | 1.04 ± 0.12 a | 0.99 ± 0.16 a | 0.71 ± 0.18 a | 0.75 ± 0.11 a |
| Na in aboveground biomass (mg g−1) | 1.57 ± 0.10 a | 6.87 ± 0.74 a | 9.01 ± 1.04 ab | 16.66 ± 2.65 b | 15.83 ± 1.95 b |
| Na in belowground biomass (mg g−1) | 2.46 ± 0.21 a | 8.13 ± 0.49 ab | 10.27 ± 1.22 b | 13.01 ± 1.57 b | 13.50 ± 1.20 b |
| Na in belowground:aboveground ratio | 1.57 ± 0.14 a | 1.27 ± 0.10 ab | 1.17 ± 0.13 ab | 0.91 ± 0.13 b | 0.88 ± 0.16 b |
| Chlorophyll content | 34.31 ± 0.60 a | 32.64 ± 0.88 a | 31.05 ± 1.37 ab | 24.66 ± 1.44 c | 26.31 ± 1.85 bc |
| Photosynthetic rate(µmol m−2 s−1) | 7.18 ± 0.82 a | 4.52 ± 0.36 ab | 3.63 ± 0.55 bc | 4.15 ± 0.91 ac | 2.35 ± 0.55 c |
| Transpiration rate(mmol m−2 s−1) | 1.87 ± 0.19 a | 0.90 ± 0.05 ab | 0.648 ± 0.08 bc | 0.46 ± 0.05 c | 0.50 ± 0.04 c |
| Stomatal conductance(mmol m−2 s−1) | 67.85 ± 7.79 a | 28.14 ± 1.74 ab | 19.96 ± 2.65 bc | 15.10 ± 1.81 c | 15.85 ± 1.19 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glaeser, L.C.; House, M.; Vitt, D.H. Reclaiming to Brackish Wetlands in the Alberta Oil Sands: Comparison of Responses to Sodium Concentrations by Carex atherodes and Carex aquatilis. Plants 2021, 10, 1511. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081511
Glaeser LC, House M, Vitt DH. Reclaiming to Brackish Wetlands in the Alberta Oil Sands: Comparison of Responses to Sodium Concentrations by Carex atherodes and Carex aquatilis. Plants. 2021; 10(8):1511. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081511
Chicago/Turabian StyleGlaeser, Lilyan C., Melissa House, and Dale H. Vitt. 2021. "Reclaiming to Brackish Wetlands in the Alberta Oil Sands: Comparison of Responses to Sodium Concentrations by Carex atherodes and Carex aquatilis" Plants 10, no. 8: 1511. https://0-doi-org.brum.beds.ac.uk/10.3390/plants10081511