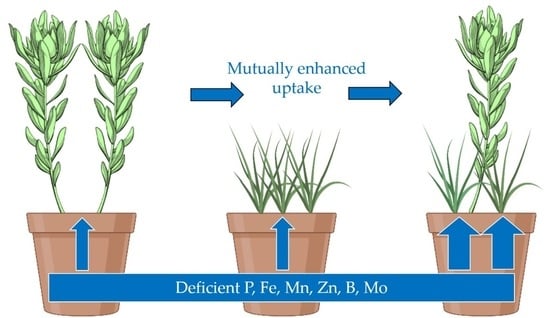
Plant Species Complementarity in Low-Fertility Degraded Soil
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Elements | Grevillea barklyana | Protea neriifolia | Grevillea Robin Hood | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alone | With Rg | With Cf | With Tg | Alone | With Rg | With Cf | With Tg | Alone | With Cf | ||
| P | 1st | 1010a | 1100a | 1170a | 1120a | 870a | 977a | 667a | 650a | 985a | 1370a |
| 2nd | 710a | 910a | 1430b | 754a | 642a | 739ab | 1140b | 696a | 630a | 1490b | |
| K | 1st | 4020a | 4450ab | 4790b | 4240a | 4900a | 4630a | 6170a | 5050a | 7600a | 7570a |
| 2nd | 4350a | 3950a | 4320a | 4270a | 7920a | 5700a | 8270a | 7340a | 6070a | 5640a | |
| Ca | 1st | 10,100a | 10,300a | 10,800a | 10,900a | 13,600a | 10,200b | 10,700b | 10,000b | 11,400a | 11,800a |
| 2nd | 9270a | 11,200a | 10,200a | 10,300a | 11,590a | 8180b | 9370b | 8650b | 9210a | 10,610a | |
| Mg | 1st | 1880a | 2030a | 2590b | 2040a | 2110a | 1660a | 1750a | 1610a | 1780a | 1910a |
| 2nd | 1050a | 1220ab | 1320b | 1150a | 1130a | 1380a | 1310a | 1290a | 1170a | 1320a | |
| S | 1st | 1290a | 1360ab | 1590b | 1330ab | 1620a | 1150a | 946a | 901a | 1620a | 1830a |
| 2nd | 812a | 1000a | 1360b | 854a | 1300ab | 923b | 1350a | 1020ab | 940a | 1280a | |
| Fe | 1st | 115ab | 110ab | 151a | 80.1b | 356a | 192b | 265ab | 233ab | 98.6a | 112a |
| 2nd | 54.9a | 58.3a | 52.4a | 93.8b | 33.3a | 32.5a | 36.7a | 46.1a | 68.3a | 49.5a | |
| Mn | 1st | 637a | 694a | 843a | 636a | 347a | 262a | 235a | 231a | 611a | 708a |
| 2nd | 429a | 926b | 679c | 643c | 325a | 216ab | 219ab | 207b | 398a | 910b | |
| Zn | 1st | 16.1a | 15.0ac | 21.4b | 13.1c | 49.6a | 27.1a | 31.1a | 29.4a | 16.2a | 15.0a |
| 2nd | 8.26a | 14.0b | 18.7c | 11.6ab | 38.2a | 17.0b | 28.1ab | 24.1ab | 13.9a | 18.9a | |
| Cu | 1st | 17.9a | 16.6ab | 19.8a | 8.53b | 15.8a | 4.27b | 5.79b | 4.89b | 12.3a | 10.2a |
| 2nd | 4.15a | 4.27a | 4.32a | 3.82a | 5.11ab | 4.03b | 5.74a | 4.47ab | 4.55a | 5.17a | |
| B | 1st | 13.5a | 14.3a | 20.2b | 13.7a | 17.4a | 15.6ab | 14.4ab | 12.9b | 20.1a | 13.2b |
| 2nd | 11.1a | 12.1ab | 13.5b | 11.5a | 21.3a | 15.1a | 19.1a | 16.5a | 15.8a | 17.6a | |
| Mo | 1st | 0.16a | 0.16a | 0.17a | 0.13a | 0.13 | <0.02 | 0.13 | 0.12 | 0.14a | 0.12a |
| 2nd | <0.02 | 0.06 | 0.14 | 0.08 | 0.03a | 0.11a | 0.02a | 0.03a | 0.07a | 0.12b | |
| Ni | 1st | 1.49a | 1.48a | 1.08a | 1.41a | 1.86a | 1.26a | 1.76a | 1.06a | 0.97a | 1.47b |
| 2nd | 0.87a | 0.73a | 0.89a | 1.07a | 0.72a | 0.73a | 0.67a | 0.71a | 1.27a | 0.70a | |
| Element | Ryegrass | Cocksfoot | Tussock Grass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alone | With Gb | With Pn | Alone | With Gb | With Pn | With GRH | Alone | With Gb | With Pn | ||
| P | 1st | 1010a | 1430b | 1310ab | 1110a | 1190a | 1390a | 1510a | 1030a | 1060a | 1110a |
| 2nd | 477a | 675b | 635ab | 412a | 501ab | 514b | 516b | 421a | 552b | 430ab | |
| K | 1st | 9570a | 12,300b | 35,400b | 11,300a | 12,700a | 39,900b | 37,100b | 7770a | 7160a | 17,100b |
| 2nd | 17,300a | 19,400a | 17,900a | 19,900a | 22,300a | 20,500a | 23,800a | 9840a | 12,600b | 9900a | |
| Ca | 1st | 6770a | 6400a | 7630a | 6340a | 6860a | 8330b | 7300b | 2990a | 3770b | 3620ab |
| 2nd | 7170a | 6460a | 7460a | 4140a | 5760a | 6110a | 4450a | 2640a | 3120a | 2890a | |
| Mg | 1st | 3460a | 3370a | 3660a | 2830a | 2920a | 2590a | 2990a | 865a | 1000a | 1250a |
| 2nd | 2870a | 2700a | 2920a | 1170a | 1600a | 1280a | 1390a | 684a | 660a | 866a | |
| S | 1st | 3190ab | 2760b | 4030a | 2870a | 3280a | 3310a | 3130a | 2230a | 1600a | 2320a |
| 2nd | 2140a | 2540a | 3220a | 1350a | 1560a | 1440a | 1760a | 1040a | 1290b | 1250ab | |
| Fe | 1st | 222a | 247a | 265a | 128a | 97.0a | 248a | 124a | 74.9a | 83.2a | 82.4a |
| 2nd | 108ab | 82.3b | 129a | 46.6a | 52.7a | 80.8a | 67.2a | 138a | 50.7a | 46.1a | |
| Mn | 1st | 94.1a | 103a | 90.1a | 287a | 351a | 364a | 320a | 105a | 141b | 121b |
| 2nd | 197a | 125a | 147a | 201a | 263a | 396a | 270a | 108a | 116a | 77.9a | |
| Zn | 1st | 50.0a | 34.2ab | 28.1b | 39.4a | 34.1a | 90.4a | 43.3a | 22.2a | 39.2a | 24.1a |
| 2nd | 43.1a | 17.3b | 24.4b | 12.9a | 20.1a | 25.8a | 27.9a | 11.0a | 15.3b | 10.7a | |
| Cu | 1st | 8.23a | 10.3a | 9.23a | 11.8a | 11.9a | 16.7a | 16.9a | 8.22a | 8.16a | 9.83a |
| 2nd | 6.11a | 5.63a | 6.07a | 4.52a | 5.59ab | 6.28b | 5.67b | 4.31a | 4.22a | 4.42a | |
| B | 1st | 8.7a | 8.07a | 7.42a | 5.79a | 6.71a | 6.67a | 7.10a | 6.27a | 5.44a | 8.56a |
| 2nd | 10.7a | 9.03a | 10.1a | 7.42a | 8.20a | 9.51a | 5.92a | 7.07a | 7.81a | 6.01a | |
| Mo | 1st | 0.41a | 0.46a | 0.43a | 0.69a | 0.58a | 0.66a | 0.89a | 0.47a | 0.53a | 0.33a |
| 2nd | 0.20a | 0.44a | 0.32a | 0.21a | 0.38a | 0.41a | 0.36a | 0.44a | 0.33ab | 0.27b | |
| Ni | 1st | 2.03a | 2.05a | 2.31a | 3.01a | 3.36a | 3.66a | 2.77a | 1.88a | 1.43a | 2.12a |
| 2nd | 2.04a | 1.75a | 1.59a | 1.56a | 1.73a | 2.50a | 3.99a | 5.36a | 1.18b | 1.51ab | |
| Species | N | P | K | Ca | Mg | S | Fe | Mn | Zn | Cu | B | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gb | 51.3a | 20,600a | 127,000a | 268,000a | 30,500a | 23,600ab | 1600ab | 12,500a | 240a | 121a | 322a | 0.58a | 25.3a |
| Gb(Rg) | 16,900a | 73,900b | 206,000a | 22,700b | 18,900a | 1130a | 16,600a | 255a | 79.5b | 226b | 0.72a | 14.1a | |
| Gb(Cf) | 40.0b | 31,600b | 94,600ab | 22,7000a | 29,200ab | 30,400b | 1150a | 15,200a | 410b | 95.0ab | 302ab | 3.03b | 20.3a |
| Gb(Tg) | 18,700a | 106,000a | 256,,000a | 28,500ab | 21,200ab | 2340b | 15,900a | 289a | 95.2ab | 286ab | 2.10b | 25.6a | |
| Pn | 11.2a | 6930ab | 77,700a | 126,000a | 12,000a | 15,200a | 358a | 3780a | 412a | 55.3a | 240a | 0.23a | 8.59a |
| Pn(Rg) | 3570a | 25,100a | 32,800b | 5390a | 3840b | 131a | 853b | 76.1b | 18.8a | 63.5b | 0.27a | 2.93a | |
| Pn(Cf) | 7.30a | 12,000b | 79,200a | 92,900ab | 12,100a | 12,600ab | 366a | 2120ab | 277ab | 55.6a | 175ab | 0.2a | 6.48a |
| Pn(Tg) | 6100ab | 66,100a | 84,500ab | 138,00a | 10,000ab | 432a | 2350ab | 220ab | 45.3a | 150ab | 0.23a | 7.74a | |
| G.RH | 29.8a | 10,800a | 112,000a | 155,000a | 20,500a | 16,200a | 1200a | 6610a | 257a | 80.9a | 279a | 0.82a | 23.3a |
| G.RH(Cf) | 26.3a | 22,400b | 90,200a | 15,9000a | 20,800a | 19,500a | 752a | 13,400b | 287a | 83.5a | 275a | 1.80b | 11.2a |
| Rg | 517a | 18,000a | 7100a | 2850a | 2230a | 106ab | 221a | 47.2a | 6.36a | 10.7a | 0.21a | 2.15a | |
| Rg(Gb) | 691a | 20,500a | 6950a | 2810a | 2690a | 85.7a | 150a | 18.7b | 5.73a | 9.59a | 0.62a | 1.89a | |
| Rg(Pn) | 752a | 21,700a | 9260a | 3580a | 3760a | 157b | 192a | 30.1ab | 7.33a | 12.8a | 0.41a | 2.00a | |
| Cf | 1050a | 49,100a | 10,300a | 2970a | 3450a | 115a | 488a | 33.8a | 11.1a | 19.1a | 0.55a | 3.96a | |
| Cf (Gb) | 899a | 38,800a | 11,300a | 2680a | 2600a | 92.7a | 451a | 31.6a | 9.56a | 16.1a | 0.72a | 3.20a | |
| CF(Pn) | 867a | 33,900a | 10,300a | 2240a | 2500a | 133a | 625a | 43.5a | 10.6a | 16.6a | 0.70a | 4.43a | |
| Cf (GRH) | 913a | 44,900a | 7410a | 2510a | 3310a | 111a | 494a | 60.8a | 10.5a | 10.2a | 0.70a | 6.84a | |
| Tg | 554a | 13,400a | 3710a | 1020ab | 1430a | 140a | 152a | 15.2a | 5.8ab | 9.96a | 0.57a | 5.36a | |
| Tg(Gb) | 596a | 13,600a | 3490a | 715a | 1400a | 51.5b | 125a | 17.0a | 4.5b | 7.87a | 0.38a | 1.35b | |
| Tg(Pn) | 767a | 19,200a | 4410a | 1460a | 2390a | 76b | 125a | 19.0a | 7.19a | 11.1a | 0.53a | 2.36b |
| Species | P | K | Ca | Mg | S | Fe | Mn | Zn | Cu | B | Mo | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gb | 20,600b | 127,000a | 268,000a | 30,500ab | 23,600b | 1600b | 12,500b | 240b | 121a | 322a | 0.58cd | 25.3a |
| Gb&Rg | 17,600b | 94,400b | 213,000a | 25,500b | 21,600b | 1210b | 16,800a | 273b | 85.2b | 236b | 1.34c | 16.0ab |
| Gb&Cf | 32,500a | 133,000a | 238,000a | 31,900a | 33,000a | 1240b | 15,600ab | 442a | 105ab | 318a | 3.75a | 23.5a |
| Gb&Tg | 19,300b | 120,000ab | 260,000a | 29,200ab | 22,600b | 2390a | 16,000ab | 306b | 99.7ab | 294ab | 2.48b | 27.0a |
| Rg | 517c | 18,000d | 7100b | 2850c | 2230c | 106c | 221c | 47.2c | 6.36c | 10.7c | 0.21d | 2.15c |
| Cf | 1050c | 49,100c | 10,300b | 2970c | 3450c | 115c | 488c | 33.8c | 11.1c | 19.1c | 0.55cd | 3.96bc |
| Tg | 554c | 13,400d | 3700b | 1020c | 1430c | 140c | 152c | 15.2c | 5.80c | 10.0c | 0.57cd | 5.36bc |
| Pn | 6930b | 77,700ab | 126,000a | 12,000a | 15,200ab | 359ab | 3780a | 412a | 55.3ab | 240a | 0.23b | 8.59abc |
| Pn&Rg | 4320bc | 46,800bc | 42,000bc | 8970ab | 7600bc | 287ab | 1050bc | 106bc | 26.2bc | 76.3bc | 0.68a | 4.94bcd |
| Pn&Cf | 12,800a | 113,000a | 103,000a | 14,400a | 15,100a | 499a | 2740ab | 320a | 66.3a | 192a | 0.90a | 10.9a |
| Pn&Tg | 6950b | 85,300ab | 88,900ab | 15,300a | 12,400ab | 524a | 2500ab | 214ab | 53.8ab | 161ab | 0.76a | 10.8ab |
| Rg | 517c | 18,000c | 7100c | 2850bc | 2230c | 106b | 221c | 47.2bc | 6.36c | 10.7c | 0.21b | 2.15d |
| Cf | 1050c | 49,100bc | 10,300c | 2970bc | 3450c | 115b | 488c | 33.8bc | 11.1c | 19.1c | 0.55ab | 3.96cd |
| Tg | 554c | 13,400c | 3700c | 1020c | 1430c | 140b | 152c | 15.2c | 5.80c | 10.0c | 0.57ab | 5.36bcd |
| G.RH | 10,800b | 112,000ab | 155,000a | 20,500a | 16,200a | 1200a | 6610b | 257a | 80.9a | 279a | 0.82b | 23.3a |
| G.RH&Cf | 23,300a | 13,5000a | 167,000a | 23,300a | 22,800a | 863a | 13,900a | 348a | 94.0a | 285a | 2.50a | 18.0ab |
| Cf | 1050c | 49,100b | 10,300b | 2970b | 3450b | 115b | 488c | 33.8b | 11.1b | 19.1b | 0.55b | 3.96b |
References
- Meister, A.; Gutierrez-Gines, M.J.; Maxfield, A.; Gaw, S.; Dickinson, N.; Horswell, J.; Robinson, B. Chemical Elements and the Quality of Manuka (Leptospermum scoparium) Honey. Foods 2021, 10, 1670. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, N.; Marmiroli, M.; Das, B.; McLaughlin, D.; Leung, D.; Robinson, B. Endemic Plants as Browse Crops in Agricultural Landscapes of New Zealand. Agroecol. Sustain. Food Syst. 2015, 39, 224–242. [Google Scholar] [CrossRef]
- Zhang, W.; Maxwell, T.M.R.; Robinson, B.; Dickinson, N. Legume nutrition is improved by neighbouring grasses. Plant Soil 2022. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1986; p. 888. [Google Scholar]
- Sturludóttir, E.; Brophy, C.; Bélanger, G.; Gustavsson, A.-M.; Jørgensen, M.; Lunnan, T.; Helgadóttir, Á. Benefits of mixing grasses and legumes for herbage yield and nutritive value in Northern Europe and Canada. Grass Forage Sci. 2014, 69, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Hector, A.; Saha, P.; Loreau, M. Biodiversity effects and transgressive overyielding. J. Plant Ecol. 2008, 1, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Nyfeler, D.; Huguenin-Elie, O.; Suter, M.; Frossard, E.; Connolly, J.; Luscher, A. Strong mixture effects among four species in fertilized agricultural grassland led to persistent and consistent transgressive overyielding. J. Appl. Ecol. 2009, 46, 683–691. [Google Scholar] [CrossRef]
- Wendling, M.; Buchi, L.; Amosse, C.; Jeangros, B.; Walter, A.; Charles, R. Specific interactions leading to transgressive overyielding in cover crop mixtures. Agric. Ecosyst. Environ. 2017, 241, 88–99. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Gliessman, S.R. Agroecology: The Ecology of Sustainable Food Systems, 3rd ed.; Taylor & Francis: Boca Raton, FL, USA, 2015; p. 371. [Google Scholar]
- Nölke, I.; Tonn, B.; Komainda, M.; Heshmati, S.; Isselstein, J. The choice of the white clover population alters overyielding of mixtures with perennial ryegrass and chicory and underlying processes. Sci. Rep. 2022, 12, 1155. [Google Scholar] [CrossRef]
- An, L.Y.; Pan, Y.H.; Wang, Z.B.; Zhu, C. Heavy metal absorption status of five plant species in monoculture and intercropping. Plant Soil 2011, 345, 237–245. [Google Scholar] [CrossRef]
- Lambers, H.; Finnegan, P.M.; Jost, R.; Plaxton, W.C.; Shane, M.W.; Stitt, M. Phosphorus nutrition in Proteaceae and beyond. Nat. Plants 2015, 1, 15109. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Massonneau, A.; Langlade, N.; Dinkelaker, B.; Hengeler, C.; Romheld, V.; Martinoia, E. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann. Bot. 2000, 85, 909–919. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Hengeler, C.; Marschner, H. Distribution and function of proteoid roots and other root clusters. Bot. Acta 1995, 108, 183–200. [Google Scholar] [CrossRef]
- Muler, A.L.; Oliveira, R.S.; Lambers, H.; Veneklaas, E.J. Does cluster-root activity benefit nutrient uptake and growth of co-existing species? Oecologia 2014, 174, 23–31. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Chen, J.; Gao, Y.B.; Ren, A.Z. Arbuscular mycorrhizal fungus identity modulates growth effects of endophyte-infected grasses on neighboring plants. Mycorrhiza 2020, 30, 663–670. [Google Scholar] [CrossRef]
- Chen, B.; Shan, X.Q.; Qian, J. Bioavailability index for quantitative evaluation of plant availability of extractable soil trace elements. Plant Soil 1996, 186, 275–283. [Google Scholar] [CrossRef]
- Gross, N.; Suding, K.N.; Lavorel, S.; Roumet, C. Complementarity as a mechanism of coexistence between functional groups of grasses. J. Ecol. 2007, 95, 1296–1305. [Google Scholar] [CrossRef]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Dickinson, N.; Baker, A.; Doronila, A.; Laidlaw, S.; Reeves, R. Phytoremediation of inorganics: Realism and synergies. Int. J. Phytoremediat. 2009, 11, 97–114. [Google Scholar] [CrossRef]
- Robinson, B.H.; Schulin, R.; Nowack, B.; Roulier, S.; Meonon, M.; Clothier, B.; Green, S.; Mills, T. Phytoremediation for the management of metal flux in contaminated sites. For. Snow Landsc. Res. 2006, 80, 221–234. [Google Scholar]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. How Plant Root Exudates Shape the Nitrogen Cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, J.; Gylfadóttir, T.; Loges, R.; Eriksen, J.; Helgadóttir, Á. Spatial and temporal variation in N transfer in grass–white clover mixtures at three Northern European field sites. Soil Biol. Biochem. 2013, 57, 654–662. [Google Scholar] [CrossRef]
- Guo, L.B.; Halliday, M.J.; Gifford, R.M. Fine root decomposition under grass and pine seedlings in controlled environmental conditions. Appl. Soil Ecol. 2006, 33, 22–29. [Google Scholar] [CrossRef]
- Prieto, I.; Birouste, M.; Zamora-Ledezma, E.; Gentit, A.; Goldin, J.; Volaire, F.; Roumet, C. Decomposition rates of fine roots from three herbaceous perennial species: Combined effect of root mixture composition and living plant community. Plant Soil 2017, 415, 359–372. [Google Scholar] [CrossRef]
- Tang, J.; Riley, W.J. On the modeling paradigm of plant root nutrient acquisition. Plant Soil 2021, 459, 441–451. [Google Scholar] [CrossRef]
- Lu, M.; Bond, W.J.; Sheffer, E.; Cramer, M.D.; West, A.G.; Allsopp, N.; February, E.C.; Chimphango, S.; Ma, Z.; Slingsby, J.A.; et al. Biome boundary maintained by intense belowground resource competition in world’s thinnest-rooted plant community. Proc. Natl. Acad. Sci. USA 2022, 119, e2117514119. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root traits with team benefits: Understanding belowground interactions in intercropping systems. Plant Soil 2021, 471, 1–26. [Google Scholar] [CrossRef]
- Lynch, J.P.; Strock, C.F.; Schneider, H.M.; Sidhu, J.S.; Ajmera, I.; Galindo-Castañeda, T.; Klein, S.P.; Hanlon, M.T. Root anatomy and soil resource capture. Plant Soil 2021, 466, 21–63. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Wang, E.Z.; Tang, Z.Y.; Rui, J.P.; Li, Y.L.; Tang, Z.X.; Dong, W.L.; Liu, X.D.; George, T.S.; Song, A.; et al. Roots and microbiome jointly drive the distributions of 17 phytohormones in the plant soil continuum in a phytohormone-specific manner. Plant Soil 2022, 470, 153–165. [Google Scholar] [CrossRef]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and Mycorrhizal Regulation of Rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef]
- Fay, P.A.; Prober, S.M.; Harpole, W.S.; Knops, J.M.H.; Bakker, J.D.; Borer, E.T.; Lind, E.M.; MacDougall, A.S.; Seabloom, E.W.; Wragg, P.D.; et al. Grassland productivity limited by multiple nutrients. Nat. Plants 2015, 1, 15080. [Google Scholar] [CrossRef]
- Franklin, H.M.; Robinson, B.H.; Dickinson, N.M. Plants for nitrogen management in riparian zones: A proposed trait-based framework to select effective species. Ecol. Manag. Restor. 2019, 20, 202–213. [Google Scholar] [CrossRef]
- Hahner, J.L.; Robinson, B.H.; Hong-Tao, Z.; Dickinson, N.M. The phytoremediation potential of native plants on New Zealand dairy farms. Int. J. Phytoremediat. 2014, 16, 719–734. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Madejón, E.; Lehto, N.J.; McLenaghen, R.D.; Horswell, J.; Dickinson, N.; Robinson, B.H. Response of a pioneering species (Leptospermum scoparium JR Forst. & G. Forst.) to heterogeneity in a low-fertility soil. Front. Plant Sci. 2019, 10, 93. [Google Scholar] [CrossRef]
- Richards, M.B.; Cowling, R.M.; Stock, W.D. Soil nutrient dynamics and community boundaries in the Fynbos vegetation of South Africa. Plant Ecol. 1997, 130, 143–153. [Google Scholar] [CrossRef]
- February, E.; Manders, P. Effects of water supply and soil type on growth, vessel diameter and vessel frequency in seedlings of three fynbos shrubs and two forest trees. S. Afr. J. Bot. 1999, 65, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Botha, P.W.; Pauw, A. Rodents and baboons reduce seed cone production of Protea neriifolia. S. Afr. J. Bot. 2017, 108, 303–307. [Google Scholar] [CrossRef]
- Heinsohn, R.D.; Pammenter, N.W. Seasonality of shoot growth and flowering in the fynbos shrub Protea neriifolia cultivated in a summer rainfall area. S. Afr. J. Bot. 1988, 54, 440–444. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H. Plant Life on the Sandplains in Southwest Australia: A Global Biodiversity Hotspot; UWA Publishing: Crawley, WA, USA, 2014; p. 332. [Google Scholar]
- Wardle, P. Vegetation of New Zealand; Cambridge University Press: Cambridge, UK, 1991; p. 672. [Google Scholar]








| Indicators | Units | Concentration | Typical Range * |
|---|---|---|---|
| pH 1 | pH Units | 5.70 | 5.70–6.20 |
| Total Nitrogen 2 | % | 0.46 | 0.30–0.60 |
| Total Carbon 3 | % | 5.80 | - |
| Organic Matter 4 | % | 10.0 | 7.00–17.0 |
| Total Phosphorus | mg kg−1 | 464 | 700–1600 |
| Olsen Phosphorus 5 | mg L−1 | 4.33 | 20.0–30.0 |
| Potassium 6 | me/100 g | 0.49 | 0.30–0.60 |
| Calcium 6 | me/100 g | 2.03 | 5.00–12.0 |
| Magnesium 7 | me/100 g | 0.60 | 0.60–1.20 |
| Sodium 7 | me/100 g | 0.05 | 0.00–0.30 |
| Sulphate Sulphur 8 | mg kg−1 | 6.43 | 10.0–20.0 |
| Iron 7 | mg L−1 | 84.0 | 500–1000 |
| Manganese 7 | mg L−1 | 3.20 | 8.00–65.0 |
| Zinc 7 | mg L−1 | 1.75 | 0.80–4.00 |
| Copper 7 | mg L−1 | 0.37 | 0.40–2.00 |
| Boron 7 | mg L−1 | 0.19 | 0.60–1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Maxwell, T.; Robinson, B.; Dickinson, N. Plant Species Complementarity in Low-Fertility Degraded Soil. Plants 2022, 11, 1370. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101370
Wei Z, Maxwell T, Robinson B, Dickinson N. Plant Species Complementarity in Low-Fertility Degraded Soil. Plants. 2022; 11(10):1370. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101370
Chicago/Turabian StyleWei, Zhang, Thomas Maxwell, Brett Robinson, and Nicholas Dickinson. 2022. "Plant Species Complementarity in Low-Fertility Degraded Soil" Plants 11, no. 10: 1370. https://0-doi-org.brum.beds.ac.uk/10.3390/plants11101370