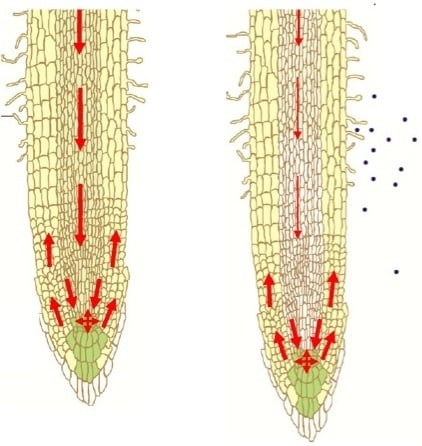
The Control of Auxin Transport in Parasitic and Symbiotic Root–Microbe Interactions
Abstract
:1. Introduction
2. Auxin Transport Carriers Regulate Plant Development
3. AUXIN RESISTANT (AUX)/LIKE-AUXIN RESISTANT (LAX) Proteins

4. PIN Proteins
5. Dynamic Repositioning of PIN Proteins during Developmental Responses
6. P-Glycoprotein/ATP-Binding Cassette Proteins
7. PILS Proteins
8. Flavonoids are Natural Auxin Transport Modulators
9. Auxin Transport Regulation during Feeding Site Establishment by Plant Parasitic Nematodes
| Gene/Protein | Host Organism | Type of Interaction | Role/Phenotype | Reference |
|---|---|---|---|---|
| AtAUX1 | Arabidopsis thaliana | Heterodera schachtii | - AUX1 expression was localized to feeding sites | [135] |
| AtPIN1 AtPIN2 | Arabidopsis thaliana | Heterodera schachtii | - Reduced H. schachtii development in infected roots of pin1/tgg1 and pin2 mutants | [122] |
| AtAUX1
AtLAX1 AtLAX2 AtLAX3 | Arabidopsis thaliana | Heterodera schachtii | - AtLAX3 interacts with cyst nematode effector Hs19C07 | [137] |
| - aux1lax1 and aux1lax1lax2lax3 mutants show reduced infection by H. schachtii | ||||
| AtPIN1
AtPIN2 AtPIN3 AtPIN4 AtPIN7 | Arabidopsis thaliana | Heterodera schachtii | - PIN1, 2, 3, 4 genes are expressed in developing feeding structures, but at different times | [138] |
| - PIN3 and PIN4 are redirected to lateral sides of feeding cells | ||||
| - Mutants of all five PIN genes show reduced infection | ||||
| AtLAX3 AtAUX1 | Arabidopsis thaliana | Meloidogyne incognita | - AtLAX3 and AtAUX1 expression induced in developing root galls | [136] |
| PtaPIN2, 4,9,12 | Poplar (Populus tremula × Populus alba) | Laccaria bicolor (Ectomycorrhiza) | - Gene expression upregulated during early interaction | [21] |
| PtaAUX6 | Poplar | Laccaria bicolor (Ectomycorrhiza) | - Gene expression upregulated during early interaction | [21] |
| AtPIN2 | Arabidopsis thaliana (nonhost) | Laccaria bicolor (Ectomycorrhiza) | - LR induction reduced by 40% in Atpin2 mutant | [21] |
| AtAUX1 | Arabidopsis thaliana (nonhost) | Tuber borchii (Ectomychorizza) | - Reduced inhibition of primary root development in Ataux1-7 mutant compared to WT | [141] |
| CgPIN1 | Casuarina glauca | Frankia inoculation | - Localized to uninfected cells adjacent to Frankia-infected cells | [142] |
| - Exports IAA into Frankia-infected cells | ||||
| CgAUX1 | Casuarina glauca | Frankia inoculation | - Localized to Frankia-infected cells | [143] |
| - Likely imports IAA into Frankia-infected cells | ||||
| DtAUX1 | Discaria trinervis | Frankia inoculation | -Localized to nodule meristem | [144] |
| MtPIN2 | Medicago truncatula | Sinorhizobium meliloti inoculation | - Expressed in peripheral vasculature in early nodule primordium | [145] |
| - Expressed at the base of mature nodule | ||||
| - Knockdown of MtPIN2 reduced nodulation | ||||
| MtPIN3 | Medicago truncatula | Sinorhizobium meliloti inoculation | - Knockdown of MtPIN3 reduced nodulation | [107] |
| MtPIN4 | Medicago truncatula | Sinorhizobium meliloti inoculation | - Knockdown of MtPIN4 reduced nodulation | [43] |
| Sinorhizobium meliloti nod factor treatment | - Increased expression after nod factor treatment | [146] | ||
| MtPIN10 | Medicago truncatula | Sinorhizobium meliloti nod factor treatment | - Increased expression after nod factor treatment | [147] |
| MtLAX1-3 | Medicago truncatula | Sinorhizobium meliloti inoculation | - Transcripts are localized to early dividing cells and to cells near the vasculature of early nodule primordium | [148] |
| LjABCB1 | Lotus japonicus | Mesorhizobium loti inoculation | - Localized to uninfected cells adjacent to Rhizobia-infected cells | [149] |
| - Exports IAA from uninfected cells into adjacent Rhizobia-infected cells |
10. Auxin Changes during Mycorrhizal Interactions
10.1. Ectomycorrhizal Symbioses
10.2. Endomycorrhizal Symbioses
11. Auxin Transport Control in Nitrogen Fixing Symbioses
11.1. Actinorhizal Symbioses

11.2. Legume-Rhizobium Symbioses

11.3. Involvement of Auxin Transport Carriers during Legume Nodulation
12. Auxin Acts as a Shoot-Root Regulator of Nodule Numbers
13. Future Questions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Giraud, E.; Perret, X.; Batut, J. Establishing nitrogen-fixing symbiosis with legumes: How many rhizobium recipes? Trends Microbiol. 2009, 17, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.; Koltai, H. Plant parasitic nematodes: Habitats, hormones, and horizontally-acquired genes. J. Plant Growth Regul. 2000, 19, 183–194. [Google Scholar] [PubMed]
- Goverse, A.; de Almeida Engler, J.; Verhees, J.; van der Krol, S.; Helder, J.; Gheysen, G. Cell cycle activation by plant parasitic nematodes. Plant Mol. Biol. 2000, 43, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Went, F.W. On growth accelerating substances in the coleoptile of Avena sativa. Proc. K. Akad. Wet 1926, 30, 10–19. [Google Scholar]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Estelle, M. The F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, N.; Dharmasiri, S.; Weijers, D.; Lechner, E.; Yamada, M.; Hobbie, L.; Ehrismann, J.S.; Jürgens, G.; Estelle, M. Plant development is regulated by a family of auxin receptor F-box proteins. Dev. Cell 2005, 9, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kepinski, S.; Leyser, O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.M.; del Pozo, J.C.; Walker, L.; Hobbie, L.; Risseeuw, E.; Banks, T.; Crosby, W.L.; Yang, M.; Ma, H.; Estelle, M. Identification of an SCF ubiquitin–ligase complex required for auxin response in Arabidopsis thaliana. Gene. Dev. 1999, 13, 1678–1691. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Wang, X.-J.; Hagen, G.; Guilfoyle, T.J. AUX/IAA proteins are active repressors, and their stability and activity are modulated by auxin. Plant Cell 2001, 13, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, J.C.; Diaz-Trivino, S.; Cisneros, N.; Gutierrez, C. The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 2006, 18, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Díaz-Triviño, S.; Abraham, Z.; Manzano, C.; Gutierrez, C.; Pozo, C.D. SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J. 2008, 53, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Jurado, S.; Abraham, Z.; Manzano, C.; López-Torrejón, G.; Pacios, L.F.; Del Pozo, J.C. The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell 2010, 22, 3891–3904. [Google Scholar] [CrossRef] [PubMed]
- Napier, R.; David, K.; Perrot-Rechenmann, C. A short history of auxin-binding proteins. Plant Mol. Biol. 2002, 49, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Klämbt, D.; Jones, A.M. Auxin-binding protein 1 does not bind auxin within the endoplasmic reticulum despite this being the predominant subcellular location for this hormone receptor. J. Biol. Chem. 1995, 270, 26962–26969. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Zhang, D.; Dai, X.; Estelle, M.; Zhao, Y. Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl. Acad. Sci. USA 2015, 112, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. Linking development to defense: Auxin in plant–pathogen interactions. Trends Plant Sci. 2009, 14, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Felten, J.; Kohler, A.; Morin, E.; Bhalerao, R.P.; Palme, K.; Martin, F.; Ditengou, F.A.; Legué, V. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 2009, 151, 1991–2005. [Google Scholar] [CrossRef] [PubMed]
- Fusconi, A. Regulation of root morphogenesis in arbuscular mycorrhizae: What role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann. Bot. 2014, 113, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; van Noorden, G.; Van Isterdael, G.; Beeckman, T.; Gheysen, G.; Mathesius, U. Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 2009, 21, 2553–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathesius, U. Goldacre paper: Auxin: At the root of nodule development? Funct. Plant Biol. 2008, 35, 651–668. [Google Scholar] [CrossRef]
- Ruiz Rosquete, M.; Barbez, E.; Kleine-Vehn, J. Cellular auxin homeostasis: Gatekeeping is housekeeping. Mol. Plant 2012, 5, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2010, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Ljung, K.; Bhalerao, R.P.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Hull, A.K.; Celenza, J.; Yamada, M.; Estelle, M.; Normanly, J.; Sandberg, G. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 2005, 17, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.V.; Johansson, A.I.; Kowalczyk, M.; Makoveychuk, A.; Wang, J.Y.; Moritz, T.; Grebe, M.; Benfey, P.N.; Sandberg, G.; Ljung, K. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 2009, 21, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. The Induction of Vascular Tissues by Auxin; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 471–492. [Google Scholar]
- Baker, D. Vascular transport of auxins and cytokinins in Ricinus. Plant Growth Regul. 2000, 32, 157–160. [Google Scholar] [CrossRef]
- Eliasson, L. Translocation of shoot-applied indolylacetic acid into the roots of Populus tremula. Physiol. Plantarum 1972, 27, 412–416. [Google Scholar] [CrossRef]
- Kramer, E.M.; Rutschow, H.L.; Mabie, S.S. AuxV: A database of auxin transport velocities. Trends Plant Sci. 2011, 16, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Friml, J. Auxin transport—Shaping the plant. Curr. Opin. Plant Biol. 2003, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Rubery, P.H.; Sheldrake, A.R. Carrier-mediated auxin transport. Planta 1974, 118, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Transport of indoleacetic acid in plant cells in relation to pH and electrical potential gradients, and its significance for polar IAA transport. New Phytol. 1975, 74, 163–172. [Google Scholar] [CrossRef]
- Goldsmith, M.H.M. The polar transport of auxin. Annu. Rev. Plant Physiol. 1977, 28, 439–478. [Google Scholar] [CrossRef]
- Parry, G.; Marchant, A.; May, S.; Swarup, R.; Swarup, K.; James, N.; Graham, N.; Allen, T.; Martucci, T.; Yemm, A.; et al. Quick on the uptake: Characterization of a family of plant auxin influx carriers. J. Plant Growth Regul. 2001, 20, 217–225. [Google Scholar] [CrossRef]
- Swarup, R.; Péret, B. AUX/LAX family of auxin influx carriers-an overview. Front. Plant Sci. 2012, 3, 225. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; Marchant, A.; Green, H.G.; May, S.T.; Ward, S.P.; Millner, P.A.; Walker, A.R.; Schulz, B.; Feldmann, K.A. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 1996, 273, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Swarup, K.; Benkova, E.; Swarup, R.; Casimiro, I.; Peret, B.; Yang, Y.; Parry, G.; Nielsen, E.; de Smet, I.; Vanneste, S.; et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008, 10, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hammes, U.Z.; Taylor, C.G.; Schachtman, D.P.; Nielsen, E. High-affinity auxin transport by the AUX1 influx carrier protein. Curr. Biol. 2006, 16, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Kerr, I.D.; Bennett, M.J. New insight into the biochemical mechanisms regulating auxin transport in plants. Biochem. J. 2007, 401, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Grebe, M.; Friml, J.; Swarup, R.; Ljung, K.; Sandberg, G.; Terlou, M.; Palme, K.; Bennett, M.J.; Scheres, B. Cell polarity signaling in Arabidopsis involves a BFA-sensitive auxin influx pathway. Curr. Biol. 2002, 12, 329–334. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Dhonukshe, P.; Swarup, R.; Bennett, M.; Friml, J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 2006, 18, 3171–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Murphy, A.S. Functional expression and characterization of Arabidopsis ABCB, AUX1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009, 59, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J.; Mravec, J.; Bouchard, R.; Blakeslee, J.J.; Abas, M.; Seifertova, D.; Wisniewska, J.; Tadele, Z.; Kubes, M.; Covanova, M.; et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006, 312, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J. Going the distance with auxin: Unravelling the molecular basis of auxin transport. Philos. T. R. Soc. B 1998, 353, 1511–1515. [Google Scholar] [CrossRef] [PubMed]
- Geldner, N.; Friml, J.; Stierhof, Y.-D.; Jurgens, G.; Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, M.; Zago, M.K.; Abas, L.; Weijers, D.; Schweighofer, A.; Meskiene, I.; Heisler, M.G.; Ohno, C.; Zhang, J.; Huang, F.; et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130, 1044–1056. [Google Scholar] [CrossRef] [PubMed]
- Zourelidou, M.; Absmanner, B.; Weller, B.; Barbosa, I.C.; Willige, B.C.; Fastner, A.; Streit, V.; Port, S.A.; Colcombet, J.; de la Fuente van Bentem, S.; et al. Auxin efflux by PIN-formed proteins is activated by two different protein kinases, D6 protein kinase and PINOID. eLife 2014, 3, e02860. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, J.J.; Bandyopadhyay, A.; Lee, O.R.; Mravec, J.; Titapiwatanakun, B.; Sauer, M.; Makam, S.N.; Cheng, Y.; Bouchard, R.; Adamec, J.; et al. Interactions among PIN-formed and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 2007, 19, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Lee, S.H.; Cho, M.; Lee, O.R.; Yoo, H.; Cho, H.-T. Differential auxin-transporting activities of PIN-formed proteins in Arabidopsis root hair cells. Plant Physiol. 2010, 153, 1046–1061. [Google Scholar] [CrossRef] [PubMed]
- Mravec, J.; Skupa, P.; Bailly, A.; Hoyerova, K.; Krecek, P.; Bielach, A.; Petrasek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.-D.; et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Feraru, E.; Vosolsobe, S.; Feraru, M.I.; Petrášek, J.; Kleine-Vehn, J. Evolution and structural diversification of PILS putative auxin carriers in plants. Front. Plant Sci. 2012, 3, 227. [Google Scholar] [CrossRef] [PubMed]
- Ranocha, P.; Dima, O.; Nagy, R.; Felten, J.; Corratgé-Faillie, C.; Novák, O.; Morreel, K.; Lacombe, B.; Martinez, Y.; Pfrunder, S.; et al. Arabidopsis WAT1 is a vacuolar auxin transport facilitator required for auxin homoeostasis. Nat. Commun. 2013, 4, 2625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, M.; Rubery, P.H. Naturally occuring auxin transport regulators. Science 1988, 241, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, K.; Blakeslee, J.J.; Titapiwatanakun, B.; Peer, W.A.; Bandyopadhyay, A.; Makam, S.N.; Lee, O.R.; Richards, E.L.; Murphy, A.S.; Sato, F.; et al. PGP4, an ATP-Binding Cassette P-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 2005, 17, 2922–2939. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.; Sovero, V.; Vincenzetti, V.; Santelia, D.; Bartnik, D.; Koenig, B.W.; Mancuso, S.; Martinoia, E.; Geisler, M. Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J. Biol. Chem. 2008, 283, 21817–21826. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bailly, A.; Zwiewka, M.; Henrichs, S.; Azzarello, E.; Mancuso, S.; Maeshima, M.; Friml, J.; Schulz, A.; Geisler, M. Arabidopsis TWISTED DWARF1 functionally interacts with auxin exporter ABCB1 on the root plasma membrane. Plant Cell 2013, 25, 202–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, P.; Dewey, E.; Friml, J.; Zhao, Y.; Snowden, K.C.; Putterill, J.; Palme, K.; Estelle, M.; Chory, J. BIG: A calossin-like protein required for polar auxin transport in Arabidopsis. Gene. Dev. 2001, 15, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Carraro, N.; Tisdale-Orr, T.E.; Clouse, R.M.; Knöller, A.S.; Spicer, R. Diversification and expression of the PIN, AUX/LAX and ABCB families of putative auxin transporters in populus. Front. Plant Sci. 2012, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Clouse, R.M.; Carraro, N. A novel phylogeny and morphological reconstruction of the PIN genes and first phylogeny of the ACC-oxidases (ACOs). Front. Plant Sci. 2014, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Forestan, C.; Farinati, S.; Varotto, S. The maize PIN gene family of auxin transporters. Front. Plant Sci. 2012, 3, a001438. [Google Scholar] [CrossRef] [PubMed]
- Nishio, S.; Moriguchi, R.; Ikeda, H.; Takahashi, H.; Takahashi, H.; Fujii, N.; Guilfoyle, T.; Kanahama, K.; Kanayama, Y. Expression analysis of the auxin efflux carrier family in tomato fruit development. Planta 2010, 232, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, E.L.; Frugoli, J. The PIN and LAX families of auxin transport genes in Medicago truncatula. Mol. Genet. Genomics 2004, 272, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Zažímalová, E.; Murphy, A.S.; Yang, H.; Hoyerová, K.; Hošek, P. Auxin transporters—why so many? Cold Spring Harb. Perspect. Biol. 2010, 2, a001552. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hilson, P.; Sedbrook, J.; Rosen, E.; Caspar, T.; Masson, P.H. The Arabidopsis thaliana AGRAVITROPIC 1 gene encodes a component of the polar-auxin-transport efflux carrier. Proc. Natl. Acad. Sci. USA 1998, 95, 15112–15117. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Yang, X.; Michniewicz, M.; Weijers, D.; Quint, A.; Tietz, O.; Benjamins, R.; Ouwerkerk, P.B.F.; Ljung, K.; Sandberg, G.; et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 2004, 306, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, J.; Xu, J.; Seifertova, D.; Brewer, P.B.; Ruzicka, K.; Blilou, I.; Roquie, D.; Benkova, E.; Scheres, B.; Friml, J. Polar PIN localization directs auxin flow in plants. Science 2006, 312, 883. [Google Scholar] [CrossRef] [PubMed]
- Geldner, N.; Anders, N.; Wolters, H.; Keicher, J.; Kornberger, W.; Muller, P.; Delbarre, A.; Ueda, T.; Nakano, A.; Jürgens, G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003, 112, 219–230. [Google Scholar] [CrossRef]
- Kleine-Vehn, J.; Huang, F.; Naramoto, S.; Zhang, J.; Michniewicz, M.; Offringa, R.; Friml, J. PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 2009, 21, 3839–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drdová, E.J.; Synek, L.; Pečenková, T.; Hála, M.; Kulich, I.; Fowler, J.E.; Murphy, A.S.; Žárský, V. The exocyst complex contributes to PIN auxin efflux carrier recycling and polar auxin transport in Arabidopsis. Plant J. 2013, 73, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Geisler, M.; Kolukisaoglu, H.Ü.; Bouchard, R.; Billion, K.; Berger, J.; Saal, B.; Frangne, N.; Koncz-Kálmán, Z.; Koncz, C.; Dudler, R.; et al. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell 2003, 14, 4238–4249. [Google Scholar] [PubMed]
- Santelia, D.; Vincenzetti, V.; Azzarello, E.; Bovet, L.; Fukao, Y.; Düchtig, P.; Mancuso, S.; Martinoia, E.; Geisler, M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005, 579, 5399–5406. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Blakeslee, J.; Lee, O.; JMravec, J.; Sauer, M.; Titapiwatanakun, B.; Makam, S.N.; Bouchard, R.; Geisler, M.; Martinoia, E.; et al. Interactions of PIN and PGP auxin transport mechanisms. Biochem. Soc. Trans. 2007, 35, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mravec, J.; Kubeš, M.; Bielach, A.; Gaykova, V.; Petrášek, J.; Skůpa, P.; Chand, S.; Benková, E.; Zažímalová, E.; Friml, J. Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 2008, 135, 3345–3354. [Google Scholar] [CrossRef] [PubMed]
- Titapiwatanakun, B.; Blakeslee, J.J.; Bandyopadhyay, A.; Yang, H.; Mravec, J.; Sauer, M.; Cheng, Y.; Adamec, J.; Nagashima, A.; Geisler, M.; et al. ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. Plant J. 2009, 57, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Barbez, E.; Kubes, M.; Rolcik, J.; Beziat, C.; Pencik, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazimalova, E. The PIN-FORMED(PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Barkawi, L.; Gardner, G.; Cohen, J.D. Transport of indole-3-butyric acid and indole-3-acetic acid in Arabidopsis hypocotyls using stable isotope labeling. Plant Physiol. 2012, 158, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Bartel, B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant 2011, 4, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Mathesius, U. The role of flavonoids in root–rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Peer, W.; Taiz, L. Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 2000, 211, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.E.; Rashotte, A.M.; Murphy, A.S.; Normanly, J.; Tague, B.W.; Peer, W.A.; Taiz, L.; Muday, G.K. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001, 126, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Pellerone, F.I.; Mathesius, U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 2006, 18, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P. Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol. Plantarum 1996, 96, 205–210. [Google Scholar] [CrossRef]
- Stenlid, G. Effects of flavonoids on the polar transport of auxins. Physiol. Plantarum 1976, 38, 262–266. [Google Scholar] [CrossRef]
- Benjamins, R.; Quint, A.; Weijers, D.; Hooykaas, P.; Offringa, R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 2001, 128, 4057–4067. [Google Scholar] [PubMed]
- Christensen, S.K.; Dagenais, N.; Chory, J.; Weigel, D. Regulation of auxin response by the protein kinase PINOID. Cell 2000, 100, 469–478. [Google Scholar] [CrossRef]
- Santner, A.A.; Watson, J.C. The WAG1 and WAG2 protein kinases negatively regulate root waving in Arabidopsis. Plant J. 2006, 45, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, P.; Edwards, K.S.; Rahman, A.; DeLong, A.; Muday, G.K. PINOID kinase regulates root gravitropism through modulation of PIN2-dependent basipetal auxin transport in Arabidopsis. Plant Physiol. 2009, 150, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Santelia, D.; Henrichs, S.; Vincenzetti, V.; Sauer, M.; Bigler, L.; Klein, M.; Bailly, A.; Lee, Y.; Friml, J.; Geisler, M.; et al. Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J. Biol. Chem. 2008, 283, 31218–31226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferté, J.; Kühnel, J.-M.; Chapuis, G.; Rolland, Y.; Lewin, G.; Schwaller, M.A. Flavonoid-related modulators of multidrug resistance: Synthesis, pharmacological activity, and structure–activity relationships. J. Med. Chem. 1999, 42, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.S.; Hoogner, K.R.; Peer, W.A.; Taiz, L. Identification, purification, and molecular cloning of N-1-naphthylphthalmic acid-binding plasma membrane-associated aminopeptidases from Arabidopsis. Plant Physiol. 2002, 128, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Brown, D.E.; Tague, B.W.; Muday, G.K.; Taiz, L.; Murphy, A.S. Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol. 2001, 126, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.; Bakos, É.; Welker, E.; Müller, M.; Goodfellow, H.R.; Higgins, C.F.; Váradi, A.; Sarkadi, B. Phosphorylation site mutations in the human multidrug transporter modulate its drug-stimulated ATPase activity. J. Biol. Chem. 1997, 272, 23165–23171. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Holman, T.; Medhurst, A.; Peer, W.; Schmuths, H.; Footitt, S.; Theodoulou, F.L.; Holdsworth, M.J. Gene expression profiling reveals defined functions of the ATP-Binding Cassette transporter COMATOSE late in phase II of germination. Plant Physiol. 2007, 143, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Verrier, P.J.; Bird, D.; Burla, B.; Dassa, E.; Forestier, C.; Geisler, M.; Klein, M.; Kolukisaoglu, Ü.; Lee, Y.; Martinoia, E.; et al. Plant ABC proteins—A unified nomenclature and updated inventory. Trends Plant Sci. 2008, 13, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, W.; de Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Vanden Bossche, R.; Karimi, M.; de Rybel, B.; Vanholme, B.; et al. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Noh, B.; Murphy, A.S.; Spalding, E.P. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 2001, 13, 2441–2454. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.; Kordbacheh, F.; Truong, T.; Hocart, C.; Djordjevic, M. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013, 238, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Han, K.; Heller, W.; Albert, A.; Dobrev, P.I.; Zažímalová, E.; Schäffner, A.R. Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. New Phytol. 2014, 201, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.M.; Geisler, M.; Bigler, L.; Ringli, C. Flavonols accumulate asymmetrically and affect auxin transport in Arabidopsis. Plant Physiol. 2011, 156, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buer, C.S.; Djordjevic, M.A. Architectural phenotypes in the transparent testa mutants of Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Wasson, A.P.; Ramsay, K.; Jones, M.G.K.; Mathesius, U. Differing requirements for flavonoids during the formation of lateral roots, nodules and root knot nematode galls in Medicago truncatula. New Phytol. 2009, 183, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Dakora, F.D.; Phillips, D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol Mol. Plant P 1996, 49, 1–20. [Google Scholar] [CrossRef]
- Hutangura, P.; Mathesius, U.; Jones, M.G.K.; Rolfe, B.G. Auxin induction is a trigger for root gall formation caused by root-knot nematodes in white clover and is associated with the activation of the flavonoid pathway. Funct. Plant Biol. 1999, 26, 221–231. [Google Scholar] [CrossRef]
- Mathesius, U.; Bayliss, C.; Weinman, J.J.; Schlaman, H.R.M.; Spaink, H.P.; Rolfe, B.G.; McCully, M.E.; Djordjevic, M.A. Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol. Plant Microbe In. 1998, 11, 1223–1232. [Google Scholar] [CrossRef]
- Svistoonoff, S.; Hocher, V.; Gherbi, H. Actinorhizal root nodule symbioses: What is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 2014, 20, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Behm, C.A.; Mathesius, U. Effectors of plant parasitic nematodes that re-program root cell development. Funct. Plant Biol. 2010, 37, 933–942. [Google Scholar] [CrossRef]
- Karczmarek, A.; Overmars, H.; Helder, J.; Goverse, A. Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Mol. Plant Pathol. 2004, 5, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Goverse, A.; Overmars, H.; Engelbertink, J.; Schots, A.; Bakker, J.; Helder, J. Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol. Plant Microbe In. 2000, 13, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, M.; Rangaswami, G. Presence of indole compound in nematode galls. Nature 1962, 194, 774–775. [Google Scholar] [CrossRef]
- Yu, P.K.; Viglierchio, D.R. Plant growth substances and parasitic nematodes. I. Root knot nematodes and tomato. Exp. Parasitol. 1964, 15, 242–248. [Google Scholar] [CrossRef]
- Gheysen, G.; Fenoll, C. Gene expression in nematode feeding sites. Annu. Rev. Phytopathol. 2002, 40, 191–219. [Google Scholar] [CrossRef] [PubMed]
- Absmanner, B.; Stadler, R.; Hammes, U.Z. Phloem development in nematode-induced feeding sites: The implications of auxin and cytokinin. Front. Plant Sci. 2013, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Ithal, N.; Recknor, J.; Nettleton, D.; Hearne, L.; Maier, T.; Baum, T.J.; Mitchum, M.G. Parallel genome-wide expression profiling of host and pathogen during soybean cyst nematode infection of soybean. Mol. Plant Microbe In. 2007, 20, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Ithal, N.; Recknor, J.; Nettleton, D.; Maier, T.; Baum, T.J.; Mitchum, M.G. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol. Plant Microbe In. 2007, 20, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Klink, V.; Overall, C.; Alkharouf, N.; MacDonald, M.; Matthews, B. A time-course comparative microarray analysis of an incompatible and compatible response by Glycine max (soybean) to Heterodera glycines (soybean cyst nematode) infection. Planta 2007, 226, 1423–1447. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Engler, J.; de Vleesschauwer, V.; Burssens, S.; Celenza, J.L.; Inzé, D.; van Montagu, M.; Engler, G.; Gheysen, G. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 1999, 11, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Hermsmeier, D.; Mazarei, M.; Baum, T.J. Differential display analysis of the early compatible interaction between soybean and the soybean cyst nematode. Mol. Plant Microbe In. 1998, 11, 1258–1263. [Google Scholar] [CrossRef]
- Wang, X.; Replogle, A.M.Y.; Davis, E.L.; Mitchum, M.G. The tobacco CEL7 gene promoter is auxin-responsive and locally induced in nematode feeding sites of heterologous plants. Mol. Plant Pathol. 2007, 8, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Furlanetto, C.; Phillips, M. The role of flavonoids produced in response to cyst nematode infection of Arabidopsis thaliana. Nematology 2007, 9, 671–677. [Google Scholar] [CrossRef]
- Péret, B.; de Rybel, B.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mazarei, M.; Lennon, K.; Puthoff, D.; Rodermel, S.; Baum, T. Expression of an Arabidopsis phosphoglycerate mutase homologue is localized to apical meristems, regulated by hormones, and induced by sedentary plant-parasitic nematodes. Plant Mol. Biol. 2003, 53, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Hammes, U.Z.; Schachtman, D.P.; Berg, R.H.; Nielsen, E.; Koch, W.; McIntyre, L.M.; Taylor, C.G. Nematode-induced changes of transporter gene expression in Arabidopsis roots. Mol. Plant Microbe In. 2005, 18, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chronis, D.; Kenning, C.; Peret, B.; Hewezi, T.; Davis, E.L.; Baum, T.J.; Hussey, R.; Bennett, M.; Mitchum, M.G. The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol. 2011, 155, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, W.; Cannoot, B.; Friml, J.; Gheysen, G. Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog. 2009, 5, e1000266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marhavý, P.; Vanstraelen, M.; de Rybel, B.; Zhaojun, D.; Bennett, M.J.; Beeckman, T.; Benková, E. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 2012, 32, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.-I.; Nakamura, S.; Fukunaga, S.; Nishimura, T.; Jenness, M.K.; Murphy, A.S.; Motose, H.; Nozaki, H.; Furutani, M.; Aoyama, T. Auxin transport sites are visualized in planta using fluorescent auxin analogs. Proc. Natl. Acad. Sci. USA 2014, 111, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Splivallo, R.; Fischer, U.; Göbel, C.; Feussner, I.; Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 2009, 150, 2018–2029. [Google Scholar] [CrossRef] [PubMed]
- Perrine-Walker, F.; Doumas, P.; Lucas, M.; Vaissayre, V.; Beauchemin, N.J.; Band, L.R.; Chopard, J.; Crabos, A.; Conejero, G.; Péret, B.; et al. Auxin carriers localization drives auxin accumulation in plant cells infected by Frankia in Casuarina glauca actinorhizal nodules. Plant Physiol. 2010, 154, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Swarup, R.; Jansen, L.; Devos, G.; Auguy, F.; Collin, M.; Santi, C.; Hocher, V.; Franche, C.; Bogusz, D.; et al. Auxin influx activity is associated with Frankia infection during actinorhizal nodule formation in Casuarina glauca. Plant Physiol. 2007, 144, 1852–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanishi, L.; Perrine-Walker, F.M.; Ndour, A.; Vayssieres, A.; Conejero, G.; Lucas, M.; Champion, A.; Laplaze, L.; Wall, L.; Svistoonoff, S. Role of auxin during intercellular infection of Discaria trinervis by Frankia. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Schabel, E.; Hughes, K.; Frugoli, J. RNAi phenotypes and the localisation of a protein::GUS fusion imply a role for Medicago truncatula PIN genes in nodulation. J. Plant Growth Regul. 2006, 25, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Plet, J.; Wasson, A.; Ariel, F.; le Signor, C.; Baker, D.; Mathesius, U.; Crespi, M.; Frugier, F. MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J. 2011, 65, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.M.; Harriott, O.T.; Moreau, R.A.; Osman, S.F.; Benson, D.R.; Jones, A.D. Hopanoid lipids compose the Frankia vesicle envelope, presumptive barrier of oxygen diffusion to nitrogenase. Proc. Natl. Acad. Sci. USA 1993, 90, 6091–6094. [Google Scholar] [CrossRef] [PubMed]
- De Billy, F.; Grosjean, C.; May, S.; Bennett, M.; Cullimore, J.V. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol. Plant Microbe In. 2001, 14, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Sugiyama, A.; Sato, S.; Tabata, S.; Yazaki, K. LjABCB1, an ATP-Binding Cassette protein specifically induced in uninfected cells of Lotus japonicus nodules. J. Plant Growth Regul. 2012, 169, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J. Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 2005, 59, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Bonfante, P. Cell wall remodeling in mycorrhizal symbiosis: A way towards biotrophism. Front. Plant Sci. 2014, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Okushima, Y.; Tasaka, M. Auxin-mediated lateral root formation in higher plants. Int. Rev. Cytol. 2007, 256, 111–137. [Google Scholar] [PubMed]
- Lavenus, J.; Goh, T.; Roberts, I.; Guyomarc’h, S.; Lucas, M.; de Smet, I.; Fukaki, H.; Beeckman, T.; Bennett, M.; Laplaze, L. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci. 2013, 18, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Marchant, A.; Bhalerao, R.P.; Beeckman, T.; Dhooge, S.; Swarup, R.; Graham, N.; Inzé, D.; Sandberg, G.; Casero, P.J.; et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001, 13, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, J.G.; Napsucialy-Mendivil, S.; Duclercq, J.; Cheng, Y.; Shishkova, S.; Ivanchenko, M.G.; Friml, J.; Murphy, A.S.; Benková, E. Auxin minimum defines a developmental window for lateral root initiation. New Phytol. 2011, 191, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.; Tagu, D. The roles of auxins and cytokinins in mycorrhizal symbioses. J. Plant Growth Regul. 2000, 19, 144–154. [Google Scholar] [PubMed]
- Gay, G.; Normand, L.; Marmeisse, R.; Sotta, B.; Debaud, J.C. Auxin overproducer mutants of Hebeloma cylindrosporum Romagnési have increased mycorrhizal activity. New Phytol. 1994, 128, 645–657. [Google Scholar] [CrossRef]
- Tagu, D.; Lapeyrie, F.; Martin, F. The ectomycorrhizal symbiosis: Genetics and development. Plant Soil 2002, 244, 97–105. [Google Scholar] [CrossRef]
- Karabaghli-Degron, C.; Sotta, B.; Bonnet, M.; Gay, G.; Le Tacon, F. The auxin transport inhibitor 2,3,5-triidobenzoic acid (TIBA) inhibits the stimulation of in vitro lateral root formation and the colonization of the tap-root cortex of norway spruce (Picea abies) seedlings by the ectomycorrhizal fungus Laccaria bicolor. New Phytol. 1998, 140, 723–733. [Google Scholar] [CrossRef]
- Tarkka, M.T.; Herrmann, S.; Wubet, T.; Feldhahn, L.; Recht, S.; Kurth, F.; Mailänder, S.; Bönn, M.; Neef, M.; Angay, O.; et al. OakContigDF159.1, a reference library for studying differential gene expression in Quercus robur during controlled biotic interactions: Use for quantitative transcriptomic profiling of oak roots in ectomycorrhizal symbiosis. New Phytol. 2013, 199, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; Adomas, A.; Li, G.; Osborne, J.; van Zyl, L.; Sederoff, R.; Finlay, R.; Stenlid, J.; Asiegbu, F. Transcriptional analysis of Pinus sylvestris roots challenged with the ectomycorrhizal fungus Laccaria bicolor. BMC Plant Biol 2008, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; Lundén, K.; Finlay, R.; Asiegbu, F.; Elfstrand, M. Expression analysis of Clavata1-like and Nodulin21-like genes from Pinus sylvestris during ectomycorrhiza formation. Mycorrhiza 2012, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kaska, D.; Myllyla, R.; Cooper, J. Auxin transport inhibitors act through ethylene to regulate dichotomous branching of lateral root meristems in pine. New Phytol. 1999, 142, 49–58. [Google Scholar] [CrossRef]
- Laajanen, K.; Vuorinen, I.; Salo, V.; Juuti, J.; Raudaskoski, M. Cloning of Pinus sylvestris SCARECROW gene and its expression pattern in the pine root system, mycorrhiza and NPA-treated short roots. New Phytol. 2007, 175, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Maillet, F.; Poinsot, V.; Andre, O.; Puech-Pages, V.; Haouy, A.; Gueunier, M.; Cromer, L.; Giraudet, D.; Formey, D.; Niebel, A.; et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011, 469, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Paszkowski, U. Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2013, 4, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Sirrenberg, A.; Göbel, C.; Grond, S.; Czempinski, N.; Ratzinger, A.; Karlovsky, P.; Santos, P.; Feussner, I.; Pawlowski, K. Piriformospora indica affects plant growth by auxin production. Physiol. Plantarum 2007, 131, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frei dit Frey, N.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef] [PubMed]
- Jentschel, K.; Thiel, D.; Rehn, F.; Ludwig-Müller, J. Arbuscular mycorrhiza enhances auxin levels and alters auxin biosynthesis in Tropaeolum majus during early stages of colonization. Physiol. Plantarum 2007, 129, 320–333. [Google Scholar] [CrossRef]
- Kaldorf, M.; Ludwig-Müller, J. AM fungi might affect the root morphology of maize by increasing indole-3-butyric acid biosynthesis. Physiol. Plantarum 2000, 109, 58–67. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Güther, M. Auxins as signals in arbuscular mycorrhiza formation. Plant Signal. Behav. 2007, 2, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Oláh, B.; Brière, C.; Bécard, G.; Dénarié, J.; Gough, C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 2005, 44, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, C.; Sawers, R.J.H.; Marti, G.; Andrés-Hernández, L.; Yang, S.-Y.; Casieri, L.; Angliker, H.; Oakeley, E.J.; Wolfender, J.-L.; Abreu-Goodger, C.; et al. Transcriptome diversity among rice root types during asymbiosis and interaction with arbuscular mycorrhizal fungi. Proc. Natl. Acad. Sci. USA 2015, 112, 6754–6759. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, M.; Gutjahr, C.; Couzigou, J.-M.; Zouine, M.; Lauressergues, D.; Timmers, A.; Audran, C.; Bouzayen, M.; Becard, G.; Combier, J.-P. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014, 166, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, M.T.; Coenen, C. Genetic evidence for auxin involvement in arbuscular mycorrhiza initiation. New Phytol. 2011, 189, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.-P.; Muller, J.; Wiemken, A.; Broughton, W.; Boller, T. Nod factors and tri-iodobenzoic acid stimulate mycorrhizal colonization and affect carbohydrate pertitioning in mycorrhizal roots of Lablab purpureum. New Phytol. 1997, 139, 361–366. [Google Scholar] [CrossRef]
- Muller, J. Mycorrhizal fungal structures are stimulated in wildtype peas and in isogenic mycorrhiza-resistant mutants by tri-iodo-benzoic acid (TIBA), an auxin-transport-inhibitor. Symbiosis 1999, 26, 379–389. [Google Scholar]
- Péret, B.; Svistoonoff, S.; Lahouze, B.; Auguy, F.; Santi, C.; Doumas, P.; Laplaze, L. A role for auxin during actinorhizal symbioses formation? Plant Signal. Behav. 2008, 3, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Becard, G.; Taylor, L.; Douds, D.; Pfeffer, P.; Doner, L. Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbioses. Mol. Plant Microbe In. 1995, 8, 252–258. [Google Scholar] [CrossRef]
- Pawlowski, K.; Demchenko, K. The diversity of actinorhizal symbiosis. Protoplasma 2012, 249, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.; Kahn, R.S.; Booth, M. Identification of indole compounds secreted by Frankia HFPArI3 in defined culture medium. Plant Soil 1989, 118, 205–209. [Google Scholar] [CrossRef]
- Hammad, Y.; Nalin, R.; Marechal, J.; Fiasson, K.; Pepin, R.; Berry, A.; Normand, P.; Domenach, A.-M. A possible role for phenyl acetic acid (PAA) on Alnus glutinosa nodulation by Frankia. Plant Soil 2003, 254, 193–205. [Google Scholar] [CrossRef]
- Wall, L.G. The actinorhizal symbiosis. J. Plant Growth Regul. 2000, 19, 167–182. [Google Scholar] [PubMed]
- Auguy, F.; Abdel-Lateif, K.; Doumas, P.; Badin, P.; Guerin, V.; Bogusz, D.; Hocher, V. Activation of the isoflavonoid pathway in actinorhizal symbioses. Funct. Plant Biol. 2011, 38, 690–696. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Vaissayre, V.; Gherbi, H.; Verries, C.; Meudec, E.; Perrine-Walker, F.; Cheynier, V.; Svistoonoff, S.; Franche, C.; Bogusz, D.; et al. Silencing of the CHALCONE SYNTHASE gene in Casuarina glauca highlights the important role of flavonoids during nodulation. New Phytol. 2013, 199, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.K.; Frost, J.W.; Long, S.R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 1986, 233, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.W.; Batley, M.; Djordjevic, M.A.; Innes, R.W.; Kuempel, P.L.; Rolfe, B.G. Flavones induce expression of nodulation genes in Rhizobium. Nature 1986, 323, 632–635. [Google Scholar] [CrossRef]
- Desbrosses, G.J.; Stougaard, J. Root nodulation: A paradigm for how plant-microbe symbiosis influences host developmental pathways. Cell Host Microbe 2011, 10, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 2008, 59, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M. Developmental biology of legume nodulation. New Phytol. 1992, 122, 211–237. [Google Scholar] [CrossRef]
- Hirsch, A.M.; Larue, T.A.; Doyle, J. Is the legume nodule a modified root or stem or an organ sui generis? Crit. Rev. Plant Sci. 1997, 16, 361–392. [Google Scholar] [CrossRef]
- Kefford, N.P.; Brockwell, J.; Zwar, J.A. The symbiotic synthesis of auxin by legumes and nodule bacteria and its role in nodule development. Aust. J. Biol. Sci. 1960, 13, 456–467. [Google Scholar]
- Pii, Y.; Crimi, M.; Cremonese, G.; Spena, A.; Pandolfini, T. Auxin and nitric oxide control indeterminate nodule formation. BMC Plant Biol 2007, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Boot, K.J.M.; van Brussel, A.A.N.; Tak, T.; Spaink, H.P.; Kijne, J.W. Lipochitin oligosaccharides from Rhizobium leguminosarum bv. viciae reduce auxin transport capacity in Vicia sativa subsp. nigra roots. Mol. Plant Microbe In. 1999, 12, 839–844. [Google Scholar] [CrossRef]
- Mathesius, U.; Schlaman, H.R.M.; Spaink, H.P.; Of Sautter, C.; Rolfe, B.G.; Djordjevic, M.A. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M.; Bhuvaneswari, T.V.; Torrey, J.G.; Bisseling, T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc. Natl. Acad. Sci. USA 1989, 86, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dickstein, R.; Cary, A.J.; Norris, J.H. The auxin transport inhibitor N-(1-naphthyl)phthalamic acid elicits pseudonodules on nonnodulating mutants of white sweetclover. Plant Physiol. 1996, 110, 501–510. [Google Scholar] [PubMed]
- Rightmyer, A.P.; Long, S.R. Pseudonodule formation by wild-type and symbiotic mutant Medicago truncatula in response to auxin transport inhibitors. Mol. Plant Microbe In. 2011, 24, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Skoog, F.; Allen, O.N. Kinetin-induced pseudonodules on tobacco roots. Am. J. Bot. 1959, 46, 610–613. [Google Scholar] [CrossRef]
- Rodriguez-Barrueco, C.; de Castro, F.B. Cytokinin-induced pseudonodules on Alnus glutinosa. Physiol. Plantarum 1973, 29, 277–280. [Google Scholar] [CrossRef]
- Scheres, B.; McKhann, H.I.; Zalensky, A.; Lobler, M.; Bisseling, T.; Hirsch, A.M. The PsENOD12 gene is expressed at two different sites in afghanistan pea pseudonodules induced by auxin transport inhibitors. Plant Physiol. 1992, 100, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Sugiyama, A.; Yazaki, K. Involvement of auxin distribution in root nodule development of Lotus japonicus. Planta 2011, 234, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacios-Bras, C.; Schlaman, H.M.; Boot, K.; Admiraal, P.; Mateos Langerak, J.; Stougaard, J.; Spaink, H. Auxin distribution in Lotus japonicus during root nodule development. Plant Mol. Biol. 2003, 52, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Suzaki, T.; Yano, K.; Ito, M.; Umehara, Y.; Suganuma, N.; Kawaguchi, M. Positive and negative regulation of cortical cell division during root nodule development in Lotus japonicus is accompanied by auxin response. Development 2012, 139, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Nizampatnam, N.R.; Baron, M.; Coppin, S.; Damodaran, S.; Adhikari, S.; Arunachalam, S.P.; Yu, O.; Subramanian, S. Ectopic expression of mir160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiol. 2013, 162, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Breakspear, A.; Liu, C.; Roy, S.; Stacey, N.; Rogers, C.; Trick, M.; Morieri, G.; Mysore, K.S.; Wen, J.; Oldroyd, G.E.D.; et al. The root hair “infectome” of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 2014, 26, 4680–4701. [Google Scholar] [CrossRef] [PubMed]
- Deinum, E.E.; Geurts, R.; Bisseling, T.; Mulder, B.M. Modeling a cortical auxin maximum for nodulation: Different signatures of potential strategies. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Van Noorden, G.E.; Ross, J.J.; Reid, J.B.; Rolfe, B.G.; Mathesius, U. Defective long-distance auxin transport regulation in the Medicago truncatula super numeric nodules mutant. Plant Physiol. 2006, 140, 1494–1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2009, 57, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Laffont, C.; Blanchet, S.; Lapierre, C.; Brocard, L.; Ratet, P.; Crespi, M.; Mathesius, U.; Frugier, F. The COMPACT ROOT ARCHITECTURE 1 gene regulates lignification, flavonoid production and polar auxin transport in Medicago truncatula. Plant Physiol. 2010, 153, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rizzo, S.; Crespi, M.; Frugier, F. The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 2006, 18, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 2015. [Google Scholar] [CrossRef] [PubMed]
- Marhavý, P.; Bielach, A.; Abas, L.; Abuzeineh, A.; Duclercq, J.; Tanaka, H.; PaYezová, M.; Petráaek, J.; Friml, J.; Kleine-Vehn, J.; et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev. Cell 2011, 21, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Marhavý, P.; Duclercq, J.; Weller, B.; Feraru, E.; Bielach, A.; Offringa, R.; Friml, J.; Schwechheimer, C.; Murphy, A.; Benková, E. Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr. Biol. 2014, 24, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Van Zeijl, A.; Op den Camp, R.H.M.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; Op den Camp, H.J.M.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [PubMed]
- Mathesius, U.; Weinman, J.J.; Rolfe, B.G.; Djordjevic, M.A. Rhizobia can induce nodules in white clover by “hijacking” mature cortical cells activated during lateral root development. Mol. Plant Microbe In. 2000, 13, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Prayitno, J.; Rolfe, B.G.; Mathesius, U. The ethylene-insensitive sickle mutant of Medicago truncatula shows altered auxin transport regulation during nodulation. Plant Physiol. 2006, 142, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Stacey, G.; Yu, O. Endogenous isoflavones are essential for the establishment of symbiosis between soybean and Bradyrhizobium japonicum. Plant J. 2006, 48, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Rípodas, C.; Via, V.D.; Aguilar, O.M.; Zanetti, M.E.; Blanco, F.A. Knock-down of a member of the ISOFLAVONOID REDUCTASE gene family impairs plant growth and nodulation in Phaseolus vulgaris. Plant Physiol. Bioch 2013, 68, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Delves, A.C.; Mathews, A.; Day, D.A.; Carter, A.S.; Carroll, B.J.; Gresshoff, P.M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986, 82, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Krusell, L.; Madsen, L.H.; Sato, S.; Aubert, G.; Genua, A.; Szczyglowski, K.; Duc, G.; Kaneko, T.; Tabata, S.; de Bruijn, F.; et al. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature 2002, 420, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Searle, I.R.; Men, A.E.; Laniya, T.S.; Buzas, D.M.; Iturbe-Ormaetxe, I.; Carroll, B.J.; Gresshoff, P.M. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science 2003, 299, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Elise, S.; Etienne-Pascal, J.; de Fernanda, C.-N.; Gérard, D.; Julia, F. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 2005, 58, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.-H.; Gresshoff, P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat Commun 2013, 4, 2191. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-derived cytokinins systemically regulate root nodulation. Nat. Commun. 2014, 5, 4983. [Google Scholar] [CrossRef] [PubMed]
- Penmetsa, R.V.; Frugoli, J.A.; Smith, L.S.; Long, S.R.; Cook, D.R. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 2003, 131, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.J.; McNeil, D.L.; Gresshoff, P.M. A supernodulation and nitrate-tolerant symbiotic (nts) soybean mutant. Plant Physiol. 1985, 78, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.E.; Ferguson, B.J.; Gresshoff, P.M. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol. Plant Microbe In. 2011, 24, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Watt, M.; Mathesius, U. The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiol. 2012, 159, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Araya, T.; Miyamoto, M.; Wibowo, J.; Suzuki, A.; Kojima, S.; Tsuchiya, Y.N.; Sawa, S.; Fukuda, H.; von Wirén, N.; Takahashi, H. CLE-CLAVATA1 peptide-receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proc. Natl. Acad. Sci. USA 2014, 111, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, F.; Zhang, F.; Mi, G. Auxin transport from shoot to root is involved in the response of lateral root growth to localized supply of nitrate in maize. Plant Sci 2005, 169, 894–900. [Google Scholar] [CrossRef]
- Tamaki, V.; Mercier, H. Cytokinins and auxin communicate nitrogen availability as long-distance signal molecules in pineapple (Ananas comosus). J. Plant Physiol. 2007, 164, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.; Brady, S.; Muday, G. Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol. 1998, 118, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Varma Penmetsa, R.; Uribe, P.; Anderson, J.; Lichtenzveig, J.; Gish, J.-C.; Nam, Y.W.; Engstrom, E.; Xu, K.; Sckisel, G.; Pereira, M.; et al. The Medicago truncatula ortholog of Arabidopsis EIN2, SICKLE, is a negative regulator of symbiotic and pathogenic microbial associations. Plant J. 2008, 55, 580–595. [Google Scholar] [CrossRef] [PubMed]
- Champion, A.; Lucas, M.; Tromas, A.; Vaissayre, V.; Crabos, A.; Diédhiou, I.; Prodjinoto, H.; Moukouanga, D.; Pirolles, E.; Cissoko, M.; et al. Inhibition of auxin signaling in Frankia species-infected cells in Casuarina glauca nodules leads to increased nodulation. Plant Physiol. 2015, 167, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Meixner, C.; Ludwig-Müller, J.; Miersch, O.; Gresshoff, P.; Staehelin, C.; Vierheilig, H. Lack of mycorrhizal autoregulation and phytohormonal changes in the supernodulating soybean mutant nts1007. Planta 2005, 222, 709–715. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, J.L.P.; Perrine-Walker, F.; Wasson, A.P.; Mathesius, U. The Control of Auxin Transport in Parasitic and Symbiotic Root–Microbe Interactions. Plants 2015, 4, 606-643. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030606
Ng JLP, Perrine-Walker F, Wasson AP, Mathesius U. The Control of Auxin Transport in Parasitic and Symbiotic Root–Microbe Interactions. Plants. 2015; 4(3):606-643. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030606
Chicago/Turabian StyleNg, Jason Liang Pin, Francine Perrine-Walker, Anton P. Wasson, and Ulrike Mathesius. 2015. "The Control of Auxin Transport in Parasitic and Symbiotic Root–Microbe Interactions" Plants 4, no. 3: 606-643. https://0-doi-org.brum.beds.ac.uk/10.3390/plants4030606