Facile Fabrication of Macroscopic Self-Standing Ni or Co-doped MnO2 Architectures as Catalysts for Propane Oxidation
Abstract
:1. Introduction
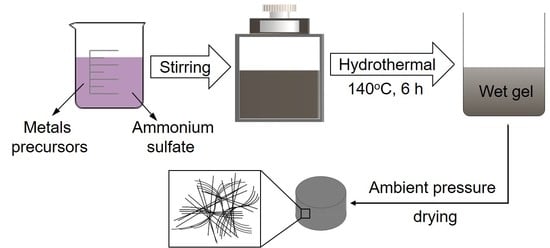
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.C.; Shim, W.G. Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B Environ. 2010, 98, 180–185. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Wang, Y.; Gong, X.; Lu, G. A highly effective Ni-modified MnOx catalyst for total oxidation of propane: The promotional role of nickel oxide. Rsc. Adv. 2016, 6, 50228–50237. [Google Scholar] [CrossRef]
- Pahalagedara, L.; Kriz, D.A.; Wasalathanthri, N.; Weerakkody, C.; Meng, Y.; Dissanayake, S.; Pahalagedara, M.; Luo, Z.; Suib, S.L.; Nandi, P.; et al. Benchmarking of manganese oxide materials with CO oxidation as catalysts for low temperature selective oxidation. Appl. Catal. B Environ. 2017, 204, 411–420. [Google Scholar] [CrossRef]
- Pahalagedara, L.R.; Dharmarathna, S.; King’ondu, C.K.; Pahalagedara, M.N.; Meng, Y.T.; Kuo, C.H.; Suib, S.L. Microwave-assisted hydrothermal synthesis of alpha-MnO2: Lattice expansion via rapid temperature ramping and framework substitution. J. Phy. Chem. C 2014, 118, 20363–20373. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, H.; Wang, L.; Zhang, Y.; Chen, C.; Hu, H.; Li, G.; Zhang, Y.; Ma, Y.; Zhang, J. Cobalt-doped K-OMS-2 nanofibers: A novel and efficient water-tolerant catalyst for the oxidation of carbon monoxide. ChemCatChem 2017, 9, 1163–1167. [Google Scholar] [CrossRef]
- Crespo-Biel, O.; Ravoo, B.J.; Reinhoudt, D.N.; Huskens, J. Noncovalent nanoarchitectures on surfaces: From 2D to 3D nanostructures. J. Mater. Chem. 2006, 16, 3997–4021. [Google Scholar] [CrossRef]
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Hui, J.F.; Wang, P.P.; Hu, S.; Xu, B.; Xiang, G.L.; Zhuang, J.; Lü, X.Q.; Wang, X. Alpha-MnO2 nanowires as building blocks for the construction of 3D macro-assemblies. Chem. Comm. 2012, 48, 5925–5927. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Jung, H.Y.; Fang, W.; Dresselhaus, M.S.; Kong, J. A facile methodology for the production of in situ inorganic nanowire hydrogels/aerogels. Nano Lett. 2014, 14, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, D.; Guo, X.; Fang, S.; Wang, L.; Xing, Y.; Suib, S.L. Robust macroscopic 3D sponges of manganese oxide molecular sieves. Chem. Eur. J. 2017, 23, 16213–16218. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.; Zhang, P.; Yang, Y.; Zhu, L.; Wang, J.; Liu, F. MnO2 framework for instantaneous mineralization of carcinogenic airborne formaldehyde at room temperature. ACS Catal. 2017, 7, 1057–1067. [Google Scholar] [CrossRef]
- Chen, L.; Ding, J.; Jia, J.; Ran, R.; Zhang, C.; Song, X. Cobalt-doped MnO2 nanofibers for enhanced propane oxidation. Acs Appl. Nano Mater. 2019, 2, 4417–4426. [Google Scholar] [CrossRef]
- Chen, L.; Ding, J.; Jia, J.; Ran, R.; Zhang, C.; Song, X. Nickel doping MnO2 with abundant surface pits as highly efficient catalysts for propane deep oxidation. Chem. Eng. J. 2019, 369, 1129–1137. [Google Scholar] [CrossRef]
- Fuchigami, T.; Kimata, R.; Haneda, M.; Kakimoto, K.I. Complex Three-dimensional Co3O4 nano-raspberry: Highly stable and active low-temperature CO oxidation catalyst. Nanomaterials 2018, 8, 662. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Chen, Y.G.; Salimi, A.R.; Mirzaei, M. Self-Assembly, Crystal structure and analysis of intermolecular interactions of the supramolecular compound based on hexamolybdochromate (III), sulfate and piperazine. J. Clust. Sci. 2011, 22, 309–318. [Google Scholar] [CrossRef]
- Jeazet, H.B.T.; Gloe, K.; Doert, T.; Kataeva, O.N.; Jäger, A.; Geipel, G.; Bernhard, G.; Büchner, B.; Gloe, K. Self-assembly of neutral hexanuclear circular copper(II) meso-helicates: Topological control by sulfate ions. Chem. Commun. 2010, 46, 2373–2375. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qiu, S.; You, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Lu, G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane. Appl. Catal. B Environ. 2018, 225, 110–120. [Google Scholar] [CrossRef]





| T10 | Surface Area (m2 g−1) | TOF × 1010 (mol m−2 s−1) | |
|---|---|---|---|
| MnO2 | 239 | 70.8 | 4.20 |
| Ni-MnO2 | 223 | 67.8 | 4.53 |
| Co-MnO2 | 229 | 59.5 | 5.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Song, X. Facile Fabrication of Macroscopic Self-Standing Ni or Co-doped MnO2 Architectures as Catalysts for Propane Oxidation. Technologies 2019, 7, 81. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies7040081
Chen L, Song X. Facile Fabrication of Macroscopic Self-Standing Ni or Co-doped MnO2 Architectures as Catalysts for Propane Oxidation. Technologies. 2019; 7(4):81. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies7040081
Chicago/Turabian StyleChen, Long, and Xiping Song. 2019. "Facile Fabrication of Macroscopic Self-Standing Ni or Co-doped MnO2 Architectures as Catalysts for Propane Oxidation" Technologies 7, no. 4: 81. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies7040081