In-Ear Electrode EEG for Practical SSVEP BCI
Abstract
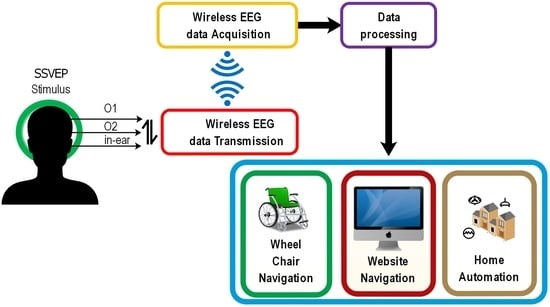
:1. Introduction
2. Methodology
2.1. Hardware Design
2.2. Data Acquisition
2.3. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liyanage, S.R.; Bhatt, C. Wearable electroencephalography technologies for brain–computer interfacing. In Wearable and Implantable Medical Devices; Dey, N., Ashour, A.S., James Fong, S., Bhatt, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 7, pp. 55–78. [Google Scholar]
- Cannard, C.; Brandmeyer, T.; Wahbeh, H.; Delorme, A. Self-health monitoring and wearable neurotechnologies. In Handbook of Clinical Neurology; Ramsey, N.F., Millán, J.d.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 168, pp. 207–232. [Google Scholar]
- Mudgal, S.K.; Sharma, S.K.; Chaturvedi, J.; Sharma, A. Brain computer interface advancement in neurosciences: Applications and issues. Interdiscip. Neurosurg. 2020, 20, 100694. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, Z.; Guan, K.; Lu, Y.; Liu, G.; Zhang, T.; Niu, H. A hybrid BCI-controlled smart home system combining SSVEP and EMG for individuals with paralysis. Biomed. Signal Process. Control 2020, 56, 101687. [Google Scholar] [CrossRef]
- Sintotskiy, G.; Hinrichs, H. In-ear-EEG—A portable platform for home monitoring. J. Med Eng. Technol. 2020, 44, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Souders, L.; Sheehan, L. Demonstrating the Therapeutic Potential of Contralesional BCI Control for Motor Recovery in Chronic Stroke. Arch. Phys. Med. Rehabil. 2019, 100, e61. [Google Scholar] [CrossRef]
- de Oliveira Júnior, W.G.; de Oliveira, J.M.; Munoz, R.; de Albuquerque, V.H.C. A proposal for Internet of Smart Home Things based on BCI system to aid patients with amyotrophic lateral sclerosis. Neural Comput. Appl. 2020, 32, 11007–11017. [Google Scholar] [CrossRef]
- Erkan, E.; Akbaba, M. A study on performance increasing in SSVEP based BCI application. Eng. Sci. Technol. 2018, 21, 421–427. [Google Scholar] [CrossRef]
- Zhu, D.; Bieger, J.; Garcia Molina, G.; Aarts, R.M. A Survey of Stimulation Methods Used in SSVEP-Based BCIs. Comput. Intell. Neurosci. 2010, 2010, 702357. [Google Scholar] [CrossRef] [PubMed]
- Mouli, S.; Palaniappan, R.; Sillitoe, I.P.; Gan, J.Q. Performance analysis of multi-frequency SSVEP-BCI using clear and frosted colour LED stimuli. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 10–13 November 2013; pp. 1–4. [Google Scholar]
- Allison, B.Z.; McFarland, D.J.; Schalk, G.; Zheng, S.D.; Jackson, M.M.; Wolpaw, J.R. Towards an independent brain-computer interface using steady state visual evoked potentials. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middendorf, M.; McMillan, G.; Calhoun, G.; Jones, K.S. Brain-computer interfaces based on the steady-state visual-evoked response. IEEE Trans. Rehabil. Eng. 2000, 8, 211–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Chen, Z.; Gao, S.; Gao, X. A high-ITR SSVEP-based BCI speller. Brain-Comput. Interfaces 2014, 1, 181–191. [Google Scholar] [CrossRef]
- Kappel, S.L.; Makeig, S.; Kidmose, P. Ear-EEG Forward Models: Improved Head-Models for Ear-EEG. Front. Neurosci. 2019, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.B.; Tabar, Y.R.; Kappel, S.L.; Christensen, C.B.; Toft, H.O.; Hemmsen, M.C.; Rank, M.L.; Otto, M.; Kidmose, P. Accurate whole-night sleep monitoring with dry-contact ear-EEG. Sci. Rep. 2019, 9, 16824. [Google Scholar] [CrossRef] [PubMed]
- Athavipach, C.; Pan-ngum, S.; Israsena, P. A Wearable In-Ear EEG Device for Emotion Monitoring. Sensors 2019, 19, 4014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floriano, A.; Diez, P.F.; Freire Bastos-Filho, T. Evaluating the influence of chromatic and luminance stimuli on SSVEPs from behind-the-ears and occipital areas. Sensors 2018, 18, 615. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.I.; Han, C.H.; Choi, G.Y.; Shin, J.; Song, K.S.; Im, C.H.; Hwang, H.J. On the feasibility of using an ear-EEG to develop an endogenous brain-computer interface. Sensors 2018, 18, 2856. [Google Scholar] [CrossRef] [Green Version]
- Mikkelsen, K.B.; Kappel, S.L.; Mandic, D.P.; Kidmose, P. EEG Recorded from the Ear: Characterizing the Ear-EEG Method. Front. Neurosci. 2015, 9, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouli, S.; Palaniappan, R. DIY hybrid SSVEP-P300 LED stimuli for BCI platform using EMOTIV EEG headset. HardwareX 2020, 8, e00113. [Google Scholar] [CrossRef]
- Kuś, R.; Duszyk, A.; Milanowski, P.; Łabęcki, M.; Bierzyńska, M.; Radzikowska, Z.; Michalska, M.; Żygierewicz, J.; Suffczyński, P.; Durka, P.J. On the Quantification of SSVEP Frequency Responses in Human EEG in Realistic BCI Conditions. PLoS ONE 2013, 8, e77536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mouli, S.; Palaniappan, R. Radial photic stimulation for maximal EEG response for BCI applications. In Proceedings of the 9th International Conference on Human System Interactions (HSI), Portsmouth, UK, 6–8 July 2016; pp. 362–367. [Google Scholar]













| Participant | Stimulus Flicker Frequency (Hz) | Peak Frequency (Hz) | SNR (dB) | Bandwidth (Hz) | |||
|---|---|---|---|---|---|---|---|
| O (Avg) | In-Ear | O (Avg) | In-Ear | O (Avg) | In-Ear | ||
| P1 | 7 | 7.10 | 7.10 | 0.02 | 7.51 | 0.46 | 0.10 |
| P2 | 7 | 7.10 | 7.10 | 8.57 | 12.63 | 0.10 | 0.14 |
| P3 | 7 | 7.10 | 7.42 | 3.92 | 27.84 | 0.10 | 0.14 |
| P4 | 7 | 7.10 | 7.10 | 1.19 | 4.20 | 0.21 | 0.28 |
| P5 | 7 | 7.10 | 7.42 | 3.75 | 8.57 | 0.10 | 0.17 |
| Avg ± Std | 7.1 ± 0 | 7.22 ± 0.17 | 3.49 ± 3.29 | 12.15 ± 9.27 | 0.19 ± 0.15 | 0.16 ± 0.06 | |
| P1 | 9 | 9.10 | 9.10 | 4.96 | 3.02 | 0.17 | 0.21 |
| P2 | 9 | 9.14 | 9.12 | 3.80 | 3.86 | 1.14 | 1.32 |
| P3 | 9 | 9.03 | 8.96 | 1.78 | 16.25 | 0.53 | 0.14 |
| P4 | 9 | 9.14 | 9.16 | 11.06 | 11.06 | 0.21 | 11.55 |
| P5 | 9 | 9.35 | 9.46 | 9.39 | 7.11 | 0.14 | 0.35 |
| Avg ± Std | 9.15 ± 0.11 | 9.16 ± 0.18 | 6.19 ± 3.89 | 8.26 ± 5.47 | 0.44 ± 0.42 | 2.71 ± 4.96 | |
| P1 | 11 | 11.03 | 11.10 | 3.69 | 0.14 | 0.14 | 0.21 |
| P2 | 11 | 11.10 | 11.11 | 6.06 | 0.59 | 0.28 | 0.10 |
| P3 | 11 | 11.11 | 11.10 | 5.56 | 0.72 | 0.29 | 0.43 |
| P4 | 11 | 11.10 | 10.56 | 1.89 | 1.87 | 0.35 | 0.21 |
| P5 | 11 | 11.25 | 11.45 | 1.68 | 5.40 | 0.18 | 0.11 |
| Avg ± Std | 11.11 ± 0.08 | 11.06 ± 0.31 | 1.62 ± 2.02 | 1.74 ± 2.14 | 0.24 ± 0.08 | 0.21 ± 0.13 | |
| P1 | 13 | 13.07 | 13.10 | 1.76 | 1.09 | 0.25 | 0.35 |
| P2 | 13 | 13.1 | 13.07 | 1.09 | 0.39 | 0.35 | 0.17 |
| P3 | 13 | 13.11 | 13.10 | 5.87 | 4.77 | 0.28 | 0.35 |
| P4 | 13 | 13.07 | 13.10 | 2.78 | 1.36 | 0.18 | 0.21 |
| P5 | 13 | 13.07 | 13.12 | 2.86 | 3.02 | 0.11 | 0.1 |
| Avg ± Std | 13.08 ± 0.01 | 13.09 ± 0.01 | 2.87 ± 1.83 | 2.12 ± 1.76 | 0.23 ± 0.09 | 0.23 ± 0.11 | |
| Stimulus Frequency (Hz) | Coefficient (r) | p-Value (p) |
|---|---|---|
| 7 Hz | 0.76 | 0.03 |
| 9 Hz | 0.89 | 0.03 |
| 11 Hz | 0.79 | 0.04 |
| 13 Hz | 0.85 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouli, S.; Palaniappan, R.; Molefi, E.; McLoughlin, I. In-Ear Electrode EEG for Practical SSVEP BCI. Technologies 2020, 8, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies8040063
Mouli S, Palaniappan R, Molefi E, McLoughlin I. In-Ear Electrode EEG for Practical SSVEP BCI. Technologies. 2020; 8(4):63. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies8040063
Chicago/Turabian StyleMouli, Surej, Ramaswamy Palaniappan, Emmanuel Molefi, and Ian McLoughlin. 2020. "In-Ear Electrode EEG for Practical SSVEP BCI" Technologies 8, no. 4: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/technologies8040063